Abstract
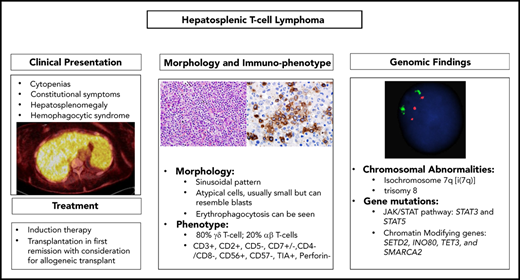
Hepatosplenic T-cell lymphoma (HSTCL) is a rare T-cell neoplasm that most commonly arises from a small subset of γ/δ T-cell receptor–expressing lymphocytes. HSTCL is more common in adolescent and young adults and has a rapidly progressive clinical course and poor outcome due to its refractoriness to conventional chemotherapy regimens. Approximately 20% of the cases arise in the background of chronic immunosuppression or immune dysregulation. Patients commonly present with constitutional symptoms, hepatic and liver enlargement, and cytopenias; hematophagocytic syndrome can also occur. The most frequent chromosomal aberrations associated with HSTCL are isochromosome 7q and trisomy 8, and most cases harbor mutations in genes involved in chromatin modification or the JAK/STAT pathway. The rarity of this disease, along with lack of nodal involvement and presenting symptoms that mimic different entities including infectious etiologies, makes this lymphoma a significant diagnostic challenge. In this review, we highlight the clinical and pathologic features of HSTCL. Moreover, we summarize the results of recent molecular studies suggesting potential targets for novel therapeutics strategies.
Introduction
Hepatosplenic T-cell lymphoma (HSTCL) is a rare subtype of peripheral T-cell lymphoma (PTCL), first described in 1981 and included as provisional entity γδ hepatosplenic T-cell lymphoma in the revised European American Lymphoma Classification in 1994.1 After the identification of rare cases with an αβ phenotype, the term “hepatosplenic T-cell lymphoma,” first used in 1990,2 has been adopted to describe this neoplasm in the World Health Organization (WHO) classification.3 HSTCL accounts for <5% of all PTCL cases with slightly over 200 cases reported in the literature. According to a retrospective study of 1314 cases of T-cell lymphoma diagnosed worldwide, the incidence of HSTCL is 1.4%.4 More recently, a prospective study, the T-cell Project, reported a similar incidence of 2%, with more cases observed in the United States compared with Europe (44% vs 25%), and extremely rare cases in other countries.5
As its name indicates, HSTCL is characterized by a proliferation of small-medium–sized mature T cells infiltrating the sinusoids of liver and spleen. Clinical presentation, histologic features, and molecular findings make this disease a unique entity among other PTCLs.
In healthy adults, γδ T cells represent <5% of circulating lymphocytes and are commonly found in mucosal sites, lymphoid and cutaneous tissues, the gastrointestinal tract, and red pulp of the spleen. They are most frequent in the spleen where they contribute 30% of the T-cell population compared with only 1% to 5% of circulating lymphocytes. They are derived from double-negative (CD4−/CD8−) thymic precursors in the bone marrow. γδ T lymphocytes are members of the innate immune system with cytotoxic features making them capable of acting as early effectors.6,7
Although the majority of cases of HSTCL occur de novo, ∼20% arise in the setting of immunosuppression or immune dysregulation, particularly autoimmune disorders, inflammatory bowel disease (IBD), hematologic malignancies, and previous solid organ transplant. Specific agents that have been implicated include common regimens used for IBD such as anti–tumor necrosis factor (anti-TNF) therapies and thiopurine immunomodulators, specifically azathioprine and 6-mercaptopurine. More than 30 cases of HSTCL involving patients with IBD have been reported since 1996.8 Risk factors for HSTCL in IBD are young age, concomitant use of anti-TNFs and thiopurines, Crohn disease as an IBD subtype, male sex, and long-term thiopurine therapy (≥2 years). Concurrent rather than single-agent TNF-α inhibitor therapy may be a stronger risk factor, but rather than being a direct result of treatment, it is likely related to the severity of the underlying disease, level of inflammatory activity, and increased immunosuppression. A case series and review of the literature reported that 89% of patients with autoimmune diseases were also treated with immunosuppressive drugs.8,9
Clinical presentation
Although HSTCL can present at any age, it occurs predominantly in young adults, with the median age of 34 years, and has a strong male predominance. Patients typically present with constitutional symptoms, abdominal discomfort due to splenomegaly and/or hepatomegaly, and, in some advanced cases, jaundice. Cytopenias are common whereas lymphadenopathy is typically absent. Thrombocytopenia is almost always present and has been shown to correlate with disease progression. Cytopenias can be due to hypersplenism, bone marrow infiltration, cytokine release, or can be immune mediated. Other laboratory findings include elevated lactate dehydrogenase (LDH), increases in levels of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase (Table 1). Although lymphocytosis is uncommon, a small population of atypical lymphocytes can be detected by flow cytometry in ∼50% of patients. In advanced cases, leukemic and blastic evolution have been reported.10 Hemophagocytic syndrome can also occur and can be associated with a rapid clinical course.11,12 Given its rarity and the absence of nodal involvement in most cases, diagnosis of HSTCL can be challenging and the disease can mimic infectious etiologies or other malignant disorders, with significant delays in diagnosis and initiation of treatment. Staging workup for HSTCL is the same as that routinely used for other non-Hodgkin lymphoma (NHL), with the exception of bone marrow aspiration and biopsy, which are an essential component of baseline evaluation in HSTCL. Splenectomy was the most common diagnostic procedures in the past. Currently, a diagnosis is made in most cases by liver and/or bone marrow biopsy. Imaging findings, including computed tomography (CT) and positron emission tomography/CT, are consistent with the clinical scenario of peculiar organ involvement (spleen and liver). CT imaging typically reveals hepatosplenomegaly without discrete lesions, whereas abnormal 18F-fluorodeoxyglucose (FDG) positron emission tomography/CT findings can include splenomegaly and/or hepatomegaly with diffuse FDG uptake and increased FDG activity in the bone marrow.13 Clinical features in cases arising in solid-organ transplant recipients are very similar, with a median age of 39 years and male predominance. Most reported cases had kidney transplants, and the median time from transplant was 6 years (range, 3-27 years).14 Delay in diagnosis is common, and all reported cases had bone marrow involvement. Prognosis is generally very poor but durable remissions have been reported with prompt reduction of immunosuppression and use of intensive chemotherapy regimens.
Summary of common clinical and pathologic features of HSTCL
| Common HSTCL features . | Patients . |
|---|---|
| Median age (range), y | 34 (5-68) |
| Previous solid organ transplant, % | 0-19 |
| Immune compromise, % | 2-27 |
| Elevated LDH, % | 55-62 |
| Abnormal LFT, % | 38-43 |
| Anemia, % | 73-84 |
| Thrombocytopenia, % | 45-95 |
| Neutropenia, % | 36-85 |
| Splenomegaly, % | 97-100 |
| Hepatomegaly, % | 40-80 |
| Adenopathy, % | 0-13 |
| Isochrome 7q, % | 25-70 |
| Trisomy 8, % | 8-53 |
| Immunophenotype | CD2+, CD3+, CD4−, CD5−, CD7+, CD8−, γδ TCR+, TIA1+ |
| Common HSTCL features . | Patients . |
|---|---|
| Median age (range), y | 34 (5-68) |
| Previous solid organ transplant, % | 0-19 |
| Immune compromise, % | 2-27 |
| Elevated LDH, % | 55-62 |
| Abnormal LFT, % | 38-43 |
| Anemia, % | 73-84 |
| Thrombocytopenia, % | 45-95 |
| Neutropenia, % | 36-85 |
| Splenomegaly, % | 97-100 |
| Hepatomegaly, % | 40-80 |
| Adenopathy, % | 0-13 |
| Isochrome 7q, % | 25-70 |
| Trisomy 8, % | 8-53 |
| Immunophenotype | CD2+, CD3+, CD4−, CD5−, CD7+, CD8−, γδ TCR+, TIA1+ |
Summary of clinical and pathologic characteristics based on largest series.17,19-21
LFT, liver function test.
Morphologic and immunophenotypic findings
The malignant cells of HSTCL are small to intermediate in size, with irregular nuclear contours, mature chromatin, inconspicuous nucleoli, a moderate amount of cytoplasm, and no granules (Figure 1). These morphologic features help differentiate HSTCL from T-cell large granular lymphocytic leukemia (LGL; T-LGL), which is characterized by lymphocytes bearing fine or coarse azurophilic granules.15
Morphologic features of different cases of HSTCL. (A) Spleen involvement with sheets of small neoplastic lymphoid cells involving cords and sinuses of spleen (hematoxylin and eosin [H&E] stain; original magnification ×600). (B) Bone marrow aspirate in a patient with progressive HSTCL (Wright-Giemsa stain; original magnification ×1000). The neoplastic T cells in this case (highlighted with thin tall arrows) are medium sized and demonstrate fine chromatic, resembling blasts. A rare neoplastic cell is demonstrating hemophagocytosis (highlighted with a thick short arrow). (C) Bone marrow core biopsy showing sinusoidal expansion by medium-sized lymphoid cells (H&E stain; original magnification ×600). (D) CD3 immunohistochemical stain in bone marrow core biopsy, highlighting the sinusoidal expansion by neoplastic T cells (original magnification ×1000).
Morphologic features of different cases of HSTCL. (A) Spleen involvement with sheets of small neoplastic lymphoid cells involving cords and sinuses of spleen (hematoxylin and eosin [H&E] stain; original magnification ×600). (B) Bone marrow aspirate in a patient with progressive HSTCL (Wright-Giemsa stain; original magnification ×1000). The neoplastic T cells in this case (highlighted with thin tall arrows) are medium sized and demonstrate fine chromatic, resembling blasts. A rare neoplastic cell is demonstrating hemophagocytosis (highlighted with a thick short arrow). (C) Bone marrow core biopsy showing sinusoidal expansion by medium-sized lymphoid cells (H&E stain; original magnification ×600). (D) CD3 immunohistochemical stain in bone marrow core biopsy, highlighting the sinusoidal expansion by neoplastic T cells (original magnification ×1000).
HSTCL typically involves the liver, spleen, and bone marrow in a sinusoidal pattern. The spleen is often markedly enlarged on gross examination (spleen weight, 550-6500 g) with a homogeneous deep brown cut surface. On microscopic evaluation, there is expansion of the red pulp with atypical lymphoid infiltration whereas the white pulp is atrophic (Figure 1A).16 However, splenectomy is rarely indicated in the modern era, as diagnosis can usually be made on bone marrow biopsy alone. The bone marrow is involved in approximately two-thirds of the patients at the time of the diagnosis and more frequently in advanced disease.10,12,16,17 The marrow infiltrate is also sinusoidal or interstitial, and, in some cases, the neoplastic T cells may resemble blasts (Figure 1B).10,12 Bone marrow infiltration may be increasingly interstitial at progression with more blastic cells. The sinusoidal pattern of infiltration can be highlighted by CD3 immunohistochemical stain (Figure 1C-D). Three patterns of infiltration in the liver have been described: sinusoidal, marked periportal infiltration with spoilage into the sinusoids, and nodular parenchymal infiltration.16 Erythrophagocytosis may be noted in sites involved by HSTCL, and, in some cases, can be associated with full-blown hemophagocytic syndrome.12,16-18 Although bone marrow involvement is common, lymphocytosis and leukemic presentations are rare, occurring in only 1% to 2% of cases.19 Similarly, lymph nodes are rarely involved at diagnosis.
By immunohistochemistry, the malignant cells are typically double negative (CD4− and CD8−), although CD8 may be expressed in some cases. They express surface CD3, CD2, and CD7 and are negative for CD5, CD1a, terminal deoxynucleotidyl transferase, and CD10.12,16,17,20,21 The majority of cases express the γδ T-cell receptor (TCR). CD56 is positive in the majority of cases, whereas CD57 is usually negative.12 Cytotoxic granule–associated markers TIA-1 and granzyme M are typically positive whereas perforin and granzyme B are negative, consistent with a nonactivated cytotoxic phenotype (Table 2). HSTCL is not an Epstein-Barr virus (EBV)-associated neoplasm, and EBV-encoded small RNA is usually negative.
Select differential diagnosis in HSTCL and distinguishing features
| Diagnosis . | Age (median/mean), y . | Common symptoms/signs . | Morphological findings . | Immunohistochemical findings . | Genomic findings . |
|---|---|---|---|---|---|
| HSTCL | 32 | B symptoms, splenomegaly, cytopenia | Atypical small-intermediate T cells, sinusoidal pattern | CD3+, CD2+, CD5−, CD7+/−, CD4−/CD8−, CD56+, CD57−, EBV− | i(7q), trisomy 8, STAT3/STAT5B mutation, CMG mutations |
| γδ T-LGL41 | 62 | Neutropenia, anemia splenomegaly +/− | LGL, sinusoidal pattern | CD3+, CD2+, CD5−/dim, CD7+, CD4−/CD8−, CD56+/−, CD57+, EBV− | STAT3/STAT5B aberrations |
| Aggressive NK-cell leukemia42 | 40 | B symptoms, splenomegaly, cytopenia, lymphadenopathy +/− | Medium-large atypical lymphoid cells | Surface CD3−, CD2+, CD5−, CD56+, CD16+, CD57−, EBV+ | Del(6q), del(11q) |
| MEITL66,67 | 59 | GI symptoms | Monomorphic medium-sized cells | CD3+, CD5−, CD7+, CD4−, CD8+, CD56+, CD103+/−, cytotoxic markers+, EBV− | Gain in 8q24 (MYC gene), STAT5B mutation |
| CAEBV45 | 19 | B symptoms, cytopenia, splenomegaly, lymphadenopathy | Small-intermediate T cells with no atypia | EBV+ in T/NK cells (Asian population) or in B cells (Western population); no phenotypic abnormalities | Rare somatic mutations of perforin |
| Diagnosis . | Age (median/mean), y . | Common symptoms/signs . | Morphological findings . | Immunohistochemical findings . | Genomic findings . |
|---|---|---|---|---|---|
| HSTCL | 32 | B symptoms, splenomegaly, cytopenia | Atypical small-intermediate T cells, sinusoidal pattern | CD3+, CD2+, CD5−, CD7+/−, CD4−/CD8−, CD56+, CD57−, EBV− | i(7q), trisomy 8, STAT3/STAT5B mutation, CMG mutations |
| γδ T-LGL41 | 62 | Neutropenia, anemia splenomegaly +/− | LGL, sinusoidal pattern | CD3+, CD2+, CD5−/dim, CD7+, CD4−/CD8−, CD56+/−, CD57+, EBV− | STAT3/STAT5B aberrations |
| Aggressive NK-cell leukemia42 | 40 | B symptoms, splenomegaly, cytopenia, lymphadenopathy +/− | Medium-large atypical lymphoid cells | Surface CD3−, CD2+, CD5−, CD56+, CD16+, CD57−, EBV+ | Del(6q), del(11q) |
| MEITL66,67 | 59 | GI symptoms | Monomorphic medium-sized cells | CD3+, CD5−, CD7+, CD4−, CD8+, CD56+, CD103+/−, cytotoxic markers+, EBV− | Gain in 8q24 (MYC gene), STAT5B mutation |
| CAEBV45 | 19 | B symptoms, cytopenia, splenomegaly, lymphadenopathy | Small-intermediate T cells with no atypia | EBV+ in T/NK cells (Asian population) or in B cells (Western population); no phenotypic abnormalities | Rare somatic mutations of perforin |
CAEBV, chronic active EBV disease; CMG, chromatin-modifying gene; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma.
The neoplastic T cells of HSTCL typically arise from a subset of γδ TCR-expressing lymphocytes, and are mostly commonly derived from the Vδ1 subset. However, ∼20% can express the αβ TCR. The αβ variants occur more commonly in women and in patients over the age of 50 years, and have been associated with worse prognosis.12,16 TCR β (TCRB) and λ (TCRG) genes are usually rearranged in HSTCL.2,16 In a study by Macon et al, TCRG was rearranged in all γδ HSTCLs and 75% of αβ HSTCLs.16 Conversely, TCRB was rearranged in all αβ HSTCLs and only 62% of γδ HSTCLs. It is important to note that most γδ HSTCLs contain nonproductive rearranged β-chains; similarly, the αβ cases contain γ-chains. Thus, the molecular clonality studies cannot be used to define the T-cell subtype (αβ vs γδ) from which the lymphoma arose.
Cytogenetic and molecular findings
Isochromosome 7q [i(7q)] and trisomy 8 are the most common chromosomal abnormalities in HSTCL, observed in up to 63% and 50% of cases, respectively, and frequently cooccurring.17 Other less common alterations include: ring chromosome 7 leading to 7q amplification, loss of Y chromosome, loss of chromosome 10q (19%), and gains in chromosome 1q (13%).22 It has been suggested that the i(7q) is the primary genetic event and others such as trisomy 8 and 7q amplification are secondary events that are acquired during progression.23,24 The role of i(7q) in pathogenesis of HSTCL is not well understood but it is postulated to be due to the altered expression of the genes on chromosome 7. Both i(7q) and ring chromosome 7 are associated with loss of 7p and amplification of 7q. A recent combined transcriptome analysis and array comparative genomic hybridization of HSTCL cases with i(7q) showed that loss of 7p22.1p14.1 was not associated with underexpression of any tumor-suppressor genes in this region, but rather increased expression of CHN2 and the encoded β2 chimerin.25 This study also found gain/amplifications of 7q22.11q31.1 and overexpression of genes in this locus including ABCB1, RUNDC3B, and PPP1R9A.25 Another study found a distinct gene-expression profile for HSTCL, distinguishing it from other T-cell lymphomas.26 The overexpressed genes encoded natural killer (NK)-related antigens including killer immunoglobulin-like receptors, and also included oncogenes (MYBL1, VAV3), cell-trafficking genes (S1PR5), a multidrug resistance 1 (MDR-1) gene, and downregulated tumor-suppressor genes such as AIM1.26
Recently, next-generation sequencing has revealed recurrent genetic alterations affecting several targetable pathways including genes involved in epigenetic regulation and JAK/STAT- and phosphatidylinositol 3-kinase (PI3K)-signaling pathways, among others. In the largest series to date, McKinney et al performed whole-exome sequencing of 68 HSTCL cases. The most frequently mutated genes included chromatin-modifying genes, comprising 62% of cases. Common epigenetic alterations included mutations in SETD2 (25%), INO80 (21%), TET3 (15%), and SMARCA2 (10%).22 The authors noted that the majority of alterations (71%) in SETD2 resulted in loss of function (nonsense and frameshift mutations), consistent with this gene having a tumor-suppressor function. Interestingly, Moffitt et al described SETD2 as the most frequently silenced gene in enteropathy-associated T-cell lymphoma, another lymphoma derived from the γδ T cell.27 The distribution of the SETD2 mutations was similar in these 2 studies, clustering in the Set2 Rpb1–interacting domain, responsible for the interaction with RNA polymerase II.22,27 SETD2 mutations have also been described in diffuse large B-cell lymphoma and acute leukemia.28,29 SETD2-mutated cells may be sensitive to WEE1 inhibition, and the current availability of WEE1 inhibitors may provide an opportunity for future investigations.30,31
McKinney et al’s study and others also confirmed recurrent somatic mutations of PIK3CD and missense mutations of STAT5B and STAT3 genes in HSTCL.32,33 Constitutive activation of the JAK/STAT pathway is commonly observed in T-cell neoplasms resulting from activating mutations of the genes in this pathway.34 STAT3 and STAT5 mutations are not unique to HSTCL. Activating mutations of STAT3 have been described in T-LGL and NK-LGL and other T-cell lymphoma subtypes.35-37 Similarly STAT5 mutations have been identified in primary cutaneous γδ T-cell lymphoma, T-cell prolymphocytic leukemia, and T-lymphoblastic leukemia.38-40 Similar to other malignancies, the mutations of STAT3 and STAT5 in HSTCL often occur in the SRC homology 2 domains, leading to constitutive activation of the protein.22,32 Other recurrent driver mutations were observed in TP53, UBR5, and IDH2.
Together, these data highlight important molecular abnormalities and may support future studies of therapies targeting recurrently altered pathways, such as epigenetic alterations and JAK/STAT and PI3K pathways (Table 3). Notably, preclinical data showed that the concomitant use of a STAT5B inhibitor and a PI3K δ inhibitor resulted in further reduction of cellular viability, indicating synergistic activity and an opportunity for clinical translation using currently available agents.22
Select mutations in HSTCL and associated targeted therapies
| Pathway . | Class of targeting therapy . | Targeted therapies . |
|---|---|---|
| Chromatin modifiers | ||
| SETD2, INO80, TET3, and | Histone deacetylase inhibitors | Vorinostat, romidepsin |
| SMARCA2, ARID1, DNMT3A | Hypomethylating agents | Decitabine, azacitidine |
| EZH2 | EZH2 inhibitors | Tazemetostat, valemetostat, CPI1205 |
| IDH2 | IDH inhibitors | Enasidenib |
| JAK/STAT | ||
| STAT5B, STAT3 | JAK inhibitors | Ruxolitinib, fedratinib |
| PI3K | ||
| PIK3CD | PI3K inhibitors | Idelalisib, copanlisib, duvelisib, alpelisib |
| NK-cell antigens | ||
| KIR3DL2, KIR2DS2, KIR3DS1 | Killer immunoglobulin-like receptor antibodies | Lirilumab (KIR2DL1/2L3) |
| KIR2DL2 | IPH4102 (KIR3DL2) | |
| NCAM1, CD244, S1PR5, CD16 (NK-cell homing to the spleen) | N/A | N/A |
| KLR | N/A | N/A |
| Growth factors | ||
| IGFBP, AREG (amphiregulin), PDGFD | Tyrosine kinase inhibitors | Imatinib, dasatinib, ponatinib nilotinib, sunitinib, axitinib, pazopanib, regorafenib, lenvatinib, nintedanib |
| Cell-trafficking genes | ||
| S1PR5 | N/A | N/A |
| Multidrug resistance | ||
| ABCB1 | N/A | N/A |
| Tyrosine kinase | ||
| SYK | SYK inhibitor | Fostamatinib |
| Chemokines | ||
| CXCL7 (PPBP), CXCL6 | N/A | N/A |
| Cell adhesion | ||
| VCAM1, CD11d, ICAM1 | N/A | N/A |
| WNT pathway | ||
| FRZB, TCF7L2, BAMBI, TLE1, CTNNB1, APC, and FZD5 | N/A | N/A |
| Microenvironment | ||
| Hemoglobin γ, β, and α | N/A | N/A |
| DEFA1/DEFA1B/ DEFA3 CCL3 (MIP1) | ||
| Tumor suppressors | ||
| KRAS, TP53 | N/A | N/A |
| Others | ||
| CREBBP, RUNDC3B, PPP1R9A, UBR5 | N/A | N/A |
| Pathway . | Class of targeting therapy . | Targeted therapies . |
|---|---|---|
| Chromatin modifiers | ||
| SETD2, INO80, TET3, and | Histone deacetylase inhibitors | Vorinostat, romidepsin |
| SMARCA2, ARID1, DNMT3A | Hypomethylating agents | Decitabine, azacitidine |
| EZH2 | EZH2 inhibitors | Tazemetostat, valemetostat, CPI1205 |
| IDH2 | IDH inhibitors | Enasidenib |
| JAK/STAT | ||
| STAT5B, STAT3 | JAK inhibitors | Ruxolitinib, fedratinib |
| PI3K | ||
| PIK3CD | PI3K inhibitors | Idelalisib, copanlisib, duvelisib, alpelisib |
| NK-cell antigens | ||
| KIR3DL2, KIR2DS2, KIR3DS1 | Killer immunoglobulin-like receptor antibodies | Lirilumab (KIR2DL1/2L3) |
| KIR2DL2 | IPH4102 (KIR3DL2) | |
| NCAM1, CD244, S1PR5, CD16 (NK-cell homing to the spleen) | N/A | N/A |
| KLR | N/A | N/A |
| Growth factors | ||
| IGFBP, AREG (amphiregulin), PDGFD | Tyrosine kinase inhibitors | Imatinib, dasatinib, ponatinib nilotinib, sunitinib, axitinib, pazopanib, regorafenib, lenvatinib, nintedanib |
| Cell-trafficking genes | ||
| S1PR5 | N/A | N/A |
| Multidrug resistance | ||
| ABCB1 | N/A | N/A |
| Tyrosine kinase | ||
| SYK | SYK inhibitor | Fostamatinib |
| Chemokines | ||
| CXCL7 (PPBP), CXCL6 | N/A | N/A |
| Cell adhesion | ||
| VCAM1, CD11d, ICAM1 | N/A | N/A |
| WNT pathway | ||
| FRZB, TCF7L2, BAMBI, TLE1, CTNNB1, APC, and FZD5 | N/A | N/A |
| Microenvironment | ||
| Hemoglobin γ, β, and α | N/A | N/A |
| DEFA1/DEFA1B/ DEFA3 CCL3 (MIP1) | ||
| Tumor suppressors | ||
| KRAS, TP53 | N/A | N/A |
| Others | ||
| CREBBP, RUNDC3B, PPP1R9A, UBR5 | N/A | N/A |
N/A, not applicable.
Differential diagnosis
The differential diagnosis for HSTCL includes other T-cell lymphomas as well as nonneoplastic conditions. Distinguishing features of a selected number of the differential diagnosis for HSTCL are presented in Table 2. Among T-cell neoplasms, CD4−/CD8− γδ T-LGL has significant overlapping features with HSTCL. T-LGL, in contrast to HSTCL involves older individuals and has a more indolent clinical course with moderately sized spleen and no increased serum LDH. Additionally, the morphology of large granular lymphocytes and absence of i(7q) in T-LGL can help with this differential diagnosis.41 Rarely, T-LGL can present in younger patients or have a more aggressive course, making the differentiation from HSTCL challenging. Yabe et al compared various clinicopathologic criteria between HSTCL and γδ T-LGL and concluded that massive splenomegaly, sinusoidal expansion in bone marrow, and lack of azurophilic granules in lymphocytes were significantly more common in HSTCL patients.15 Notably, T-LGL also involves the sinusoids in bone marrow. However, the sinusoidal involvement of bone marrow in T-LGL is characterized by a short linear (1 single layer) array of cytotoxic T cells, rather than sinusoidal expansion like seen in HSTCL.41 The other T-cell neoplasms that should be considered in the differential diagnosis of HSTCL include T-lymphoblastic leukemia, primary cutaneous γδ T-cell lymphoma, intestinal T-cell lymphomas such as monomorphic epitheliotropic intestinal T-cell lymphoma, and EBV+ T-cell lymphoma of childhood. The differentiation from these neoplasms is less challenging, given a more distinctive clinical and immunophenotypic features. Aggressive NK-cell leukemia, a rare form of leukemia that is more common among Asians, can have several overlapping clinical features with HSTCL, including constitutional symptoms, hepatosplenomegaly, and cytopenias. The neoplastic cells have similar morphologic and immunophenotypic features as HSTCL (Table 2). However, this neoplasm is EBV+ and TCR genes are in germline configuration.42
The presenting clinical symptoms of HSTCL also have some overlap with acute viral infections and chronic active EBV infection.43 The patients with CAEBV can present with B symptoms, splenomegaly, and cytopenia. The bone marrow biopsy in these patients shows an infiltrate of EBV+ small-intermediate–sized lymphocytes (B, T, or NK cells) without significant atypia or phenotypic abnormalities.44,45 It is also very important to consider HSTCL in the differential diagnosis when evaluating patients who present with hemophagocytosis syndrome.11
Treatment
Rare subtypes of PTCLs including HSTCL are underrepresented in most registry and clinical studies, and most of the literature is obtained from small case series or single-institution retrospective studies, making it very difficult to draw conclusions about the effectiveness of different treatment modalities. Outcomes with standard anthracycline-containing induction regimens have been disappointing with variable responses, high relapse rates, and a short median survival.12,20,21,46 Two single-institution studies reported poor results with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like regimens.20,21 Belhadj et al reported on 21 cases of γδ HSTCL; the overall response rate was 73% and included 9 patients who achieved complete response (CR).21 However, despite the initial response, median survival time was only 16 months, and only 2 patients were alive at the time of the analysis. Interestingly, in all patients’ progression involved initial sites of disease and, at the time of progression, recurrent thrombocytopenia was observed. Falchook et al reported the MD Anderson Cancer Center (MDACC) experience regarding 15 cases of HSTCL.20 Among the 6 patients treated with CHOP/CHOP-like regimens, only 2 achieved a CR, which was short lived, with a median duration of only 8 months. In a limited number of patients, a more intensive regimen, fractionated cyclophosphamide, liposomal doxorubicin, vincristine, and dexamethasone (HyperCIDDDoxil), alternating with methotrexate and high-dose cytarabine, resulted in a higher rate of CR. Poor prognostic factors included male sex, failure to achieve a CR, and history of immunocompromise. All patients who received a hematopoietic stem cell transplantation (SCT) were alive and in CR at the time of the analysis, suggesting that this modality can be potentially curative. Prognosis is worse in patients with posttransplant HSTCL. Tey et al reviewed 21 patients who were managed with reduction of immunosuppression and mostly CHOP-like treatment, and the reported median survival was only 4 months.14 A few reports describe modest clinical activity of purine analogs (pentostatin, fludarabine, and cladribine) in single patients (Table 4).12,46-48 Splenectomy can be a useful therapeutic option in selected patients, particularly in patients with significant thrombocytopenia, which can limit the administration of full doses of chemotherapy.17 Overexpression of MDR-1 and pgp-1 amplification observed in HSTCL and other T-cell lymphoma subtypes can explain the chemotherapy refractoriness of these diseases and support the use of non-MDR–susceptible agents.26
Patients with relapsed or refractory disease have poor outcomes with often rapidly fatal disease in the absence of transplant. Available evidence suggests that some patients have been salvaged by incorporating therapy with alternative mechanisms of action (ie, ifosfamide and cytarabine-based regimens if CHOP-like therapy was given first line), and consolidation with transplantation or even repeat transplantation. Successful relapsed or bridging regimens included gemcitabine, carboplatin, and dexamethasone as a bridge to allogeneic transplant49 ; etoposide, methylprednisolone, high-dose cytarabine, and cisplatin; alemtuzumab; and purine analogs.50 Alemtuzumab may have efficacy in this setting given the ubiquitous expression of CD52 in HSTCL.51 A few case reports demonstrated some efficacy of alemtuzumab as a single agent or combined with a purine analog.43,50,52 However, the combination can cause prolonged cytopenias, increased risk for infection, and difficulty with mobilization in those planning autologous SCT (auto-SCT).52 Notably, in 1 of these cases, a patient was treated with cladribine and alemtuzumab and maintained continuous remission without transplantation, which was precluded due to inability to mobilize stem cells.52 In addition, there have been anecdotal reports of activity of novel agents including pralatrexate used alone or in combination with romidepsin.31,53 Several case reports have also demonstrated the efficacy of allogeneic transplant in this setting, which was effective even in patients with active disease at the time of transplant.54-57
Role of transplant in HSTCL
HSTCL has poor outcomes in the absence of transplant with 5-year survival of <10%.4 The outcomes posttransplantation are not known, and data to support transplantation are again limited to small retrospective studies and expert opinions.57 Both auto-SCT and allogeneic SCT (allo-SCT) have demonstrated feasibility and the potential for long-term responses. However, the true benefit of transplant in light of patient selection and response cannot be determined. In an analysis of 27 patients with HSTCL, SCT receipt was associated with longer event-free survival (EFS) but not overall survival (OS).12 Nevertheless, reports of a remission following donor lymphocyte infusion58 and a response following reduced immunosuppression post allo-SCT indicate a strong graft-versus-tumor effect, providing a theoretical basis for the role of transplant in this disease.59 Voss et al reported a single-center experience on 14 patients with HSTCL treated with various regimens, and observed that 7 of them remained alive with a median follow-up of 65.6 months. Among these 7 patients, 6 received alternative induction regimens such as ifosfamide, carboplatin, etoposide or ifosfamide, etoposide, high-dose cytarabine and all surviving patients received either auto-SCT or allo-SCT.46 The North American Peripheral T-Cell Lymphoma Consortium demonstrated the feasibility of allo-SCT in first remission, with 21 of 42 patients successfully completing allo-SCT.60 Notably, all but 1 of the surviving patients at the time of presentation had received allo-SCT. Similarly, a European registry–based study of 18 patients undergoing allo-SCT for HSTCL reported relatively favorable outcomes: 3-year OS and progression-free survival were 54% and 48%, respectively.61 Allogeneic transplant was typically performed in first or second remission, commonly using ifosfamide-based induction and myeloablative conditioning regimens that commonly included total-body irradiation.46,62-65 A systematic review of 44 cases demonstrated a 35% relapse rate following transplant for a 3-year relapse-free survival of 42% and OS of 56%. No cases relapsed beyond 1.5 years. Interestingly, active disease did not predict posttransplant outcomes, further highlighting the role of a graft-versus-tumor effect57,61 Overall, acute and chronic graft-versus-host disease were common, but appeared manageable, with patients reported to be alive between 12 and 86 months posttransplant. However, transplant was associated with high rates of morbidity and mortality, with a nonrelapse mortality rate as high as 68%.57
auto-SCT has also demonstrated some efficacy, particularly when following platinum-containing regimens, with long-term survival reported in several case reports and case series.8,12,16,21,46 Although it is difficult to compare outcomes in patients receiving allo- and auto-SCT due to baseline differences in patients, a report from the European Society for Bone and Marrow Transplantation, using a database that included 18 allo-SCT and 7 auto-SCT, showed that those undergoing auto-SCT did not fare well, with only 1 of 7 achieving long-term remission, compared with only 2 relapsing in the allo-SCT group.61 Similarly, in a recent analysis of 12 patients who received SCT at MDACC, there was a trend, albeit not statistically significant, toward better results with allo-SCT (median OS, not reached; median EFS, not reached) compared with auto-SCT (median OS, 58.4 months; median EFS, 43.2 months). Together, these data suggest that non-CHOP induction followed by auto- or allo-SCT in first remission may offer the best opportunity for long-term survival in transplant-eligible patients. However, severe selection bias, short follow-up times, and limited long-term data with single-digit patient outcomes complicate interpretation of these results for clinical practice. Given the rarity of the disease, a comprehensive multinational study is needed to further quantify this benefit (Table 4).
Select treatment outcomes in HSTCL (>2 patients)
| Reference . | N . | Treatment . | Transplant . | Response . | Outcomes . |
|---|---|---|---|---|---|
| Foss et al5 | 31 | Nonanthracycline 40% | auto (1) | 40% CR | Median survival 13 mo |
| Anthracycline 60% | allo (7: 4 in CR1, 3 in salvage) | 3-y OS 40% | |||
| 3-y PFS 40% | |||||
| Yabe et al12 | 27 | HyperCVAD (15) | autoSCT (7) | CR (4) | Median OS of 28.3 mo |
| CHOP-like (9) | alloSCT (5) | SCT receipt associated with longer EFS but not OS | |||
| Other (19) | All patients eventually DOD | ||||
| Pentostatin (6) | |||||
| Alemtuzumab (6) | |||||
| Belhadj et al21 | 21 | CHOP/CHOP-like (19) | autoSCT (6) | CR (11) | All patients DOD except 2 treated with platinum agents (and autoSCT) |
| Platinum/Ara-C (2) | alloSCT (2) | PR (2) | |||
| PD (6) | |||||
| PR (2) | |||||
| Falchook et al20 | 15 | CHOP/CHOP-like (6) | autoSCT (5) | 50% CR (7 of 14 receiving chemotherapy) | Median duration of CR of 8 mo |
| HyperCVIDDMTX/Ara-C (4) | alloSCT (2) | All patients who received SCT are alive and in CR | |||
| Other (4) | |||||
| Voss et al46 | 14 | CHOP (4) | autoSCT (4) | 1 CR, 2 PR, 1 PD | 7 remain alive (median f/u 65.6 mo) |
| ICE/IVAC (8) | alloSCT (8) | 4 CR, 2 PR, 2 PD | 6 of 7 alive had non-CHOP and SCT | ||
| Pentostatin/2-CDA (2) | 2 PD | ||||
| Macon et al16 | 14 | None (5) | autoSCT (2) | N/A | Median OS 6 mo |
| CHOP (2) | 11 DOD, 8 within a year of diagnosis | ||||
| HDT + auto-SCT (2) | 2 long-term remission: 1 after autoSCT, 1 after intensive regimen | ||||
| Other intensive chemotherapy (2) | |||||
| Yabe et al8 | 7 | CHOP-like (4) | auto (1) | N/A | 4 alive (f/u 1-86 mo) |
| HyperCVAD-like (3) | allo (2) | 3 of 4 alive had SCT | |||
| Hassan et al68 | 7 | CHOP (1) | None | PD (1) | All DOD |
| Saito et al69 | 3 | LSG9 (1) | None | CR (1) | All DOD <9 mo |
| CHOP + ESHAP (1) | PD (1) | ||||
| CHOP (1) | CR (1) | ||||
| Rashidi and Cashen57 | 44 | alloSCT, TBI-based conditioning 71% | allo (44) | Pre-alloSCT response | 42% 3-y RFS |
| 41% CR | 56% 3-y OS | ||||
| 43% PR | |||||
| 16% PD |
| Reference . | N . | Treatment . | Transplant . | Response . | Outcomes . |
|---|---|---|---|---|---|
| Foss et al5 | 31 | Nonanthracycline 40% | auto (1) | 40% CR | Median survival 13 mo |
| Anthracycline 60% | allo (7: 4 in CR1, 3 in salvage) | 3-y OS 40% | |||
| 3-y PFS 40% | |||||
| Yabe et al12 | 27 | HyperCVAD (15) | autoSCT (7) | CR (4) | Median OS of 28.3 mo |
| CHOP-like (9) | alloSCT (5) | SCT receipt associated with longer EFS but not OS | |||
| Other (19) | All patients eventually DOD | ||||
| Pentostatin (6) | |||||
| Alemtuzumab (6) | |||||
| Belhadj et al21 | 21 | CHOP/CHOP-like (19) | autoSCT (6) | CR (11) | All patients DOD except 2 treated with platinum agents (and autoSCT) |
| Platinum/Ara-C (2) | alloSCT (2) | PR (2) | |||
| PD (6) | |||||
| PR (2) | |||||
| Falchook et al20 | 15 | CHOP/CHOP-like (6) | autoSCT (5) | 50% CR (7 of 14 receiving chemotherapy) | Median duration of CR of 8 mo |
| HyperCVIDDMTX/Ara-C (4) | alloSCT (2) | All patients who received SCT are alive and in CR | |||
| Other (4) | |||||
| Voss et al46 | 14 | CHOP (4) | autoSCT (4) | 1 CR, 2 PR, 1 PD | 7 remain alive (median f/u 65.6 mo) |
| ICE/IVAC (8) | alloSCT (8) | 4 CR, 2 PR, 2 PD | 6 of 7 alive had non-CHOP and SCT | ||
| Pentostatin/2-CDA (2) | 2 PD | ||||
| Macon et al16 | 14 | None (5) | autoSCT (2) | N/A | Median OS 6 mo |
| CHOP (2) | 11 DOD, 8 within a year of diagnosis | ||||
| HDT + auto-SCT (2) | 2 long-term remission: 1 after autoSCT, 1 after intensive regimen | ||||
| Other intensive chemotherapy (2) | |||||
| Yabe et al8 | 7 | CHOP-like (4) | auto (1) | N/A | 4 alive (f/u 1-86 mo) |
| HyperCVAD-like (3) | allo (2) | 3 of 4 alive had SCT | |||
| Hassan et al68 | 7 | CHOP (1) | None | PD (1) | All DOD |
| Saito et al69 | 3 | LSG9 (1) | None | CR (1) | All DOD <9 mo |
| CHOP + ESHAP (1) | PD (1) | ||||
| CHOP (1) | CR (1) | ||||
| Rashidi and Cashen57 | 44 | alloSCT, TBI-based conditioning 71% | allo (44) | Pre-alloSCT response | 42% 3-y RFS |
| 41% CR | 56% 3-y OS | ||||
| 43% PR | |||||
| 16% PD |
2-CDA, cladribine; allo, allogeneic; auto, autologous; CR1, first complete response; DOD, died of disease; ESHAP, etoposide, methylprednisolone, high-dose cytarabine, cisplatin; f/u, follow-up; HyperCVAD cyclophosphamide, vincristine, doxorubicin, and dexamethasone; HyperCVIDDMTX/Ara-C, fractionated cyclophosphamide, liposomal doxorubicin, dexamethasone, vincristine, methotrexate, cytarabine; ICE, ifosfamide, carboplatin, etoposide; IVAC, ifosfamide, etoposide, cytarabine; LSG9, VEPA-B/FEPP-AB/M-FEPA; N/A, not applicable; PD, progressive disease; PFS, progression-free survival; PR, partial response; RFS, relapse-free survival; TBI total-body irradiation.
Conclusions
HSTCL is a rare highly aggressive T-cell lymphoma that poses significant diagnostic and therapeutic challenges. Due to the rapid and aggressive clinical course, HSTCL is a critical diagnosis to consider in patients presenting with cytopenias and hepatosplenomegaly, particularly in young male patients with a history of immunocompromise. To date, from the limited data available, it appears that the best therapeutic approach is non-CHOP induction therapy followed by consolidation with allogeneic or autologous (if limited donor availability) transplant. Our standard approach is to use cyclophosphamide, vincristine, doxorubicin, and dexamethasone (HyperCVAD) or HyperCVAD-like regimens, and we immediately refer our patients for consideration of high-dose therapy with stem cell support. Alternatively, platinum-, ifosfamide-, or different cytarabine-containing regimens can be used, as they have also been associated with superior results. Major progress has been made in recent years with molecular studies, which have identified mutations in oncogenic pathways that may be targets for therapy. Progress in rare diseases can only be achieved by increasing awareness and collaborations among researchers worldwide.
Acknowledgments
The authors are thankful to Yi-Hua Chen for providing the images presented in Figure 1, and Xinyan Lu for the fluorescence in situ hybridization image presented in the visual abstract.
Authorship
Contribution: B.P., P.A., and A.B. all contributed to the drafting of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara Pro, Division of Hematology/Oncology, Department of Medicine, Feinberg School of Medicine, Northwestern University, 676 N St Clair St, Suite 850, Chicago, IL 60611; e-mail: barbara.pro@nm.org.

![Morphologic features of different cases of HSTCL. (A) Spleen involvement with sheets of small neoplastic lymphoid cells involving cords and sinuses of spleen (hematoxylin and eosin [H&E] stain; original magnification ×600). (B) Bone marrow aspirate in a patient with progressive HSTCL (Wright-Giemsa stain; original magnification ×1000). The neoplastic T cells in this case (highlighted with thin tall arrows) are medium sized and demonstrate fine chromatic, resembling blasts. A rare neoplastic cell is demonstrating hemophagocytosis (highlighted with a thick short arrow). (C) Bone marrow core biopsy showing sinusoidal expansion by medium-sized lymphoid cells (H&E stain; original magnification ×600). (D) CD3 immunohistochemical stain in bone marrow core biopsy, highlighting the sinusoidal expansion by neoplastic T cells (original magnification ×1000).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/18/10.1182_blood.2019004118/1/m_bloodbld2019004118cf1.png?Expires=1769739591&Signature=x5ta1BQZVA4O6kzI8xG0riQqKmDLKuZ6pcBuzTGyfOKStFnDgBHdHG~-Kfp06v71TVrR6A0GonoUYcZABPPDuxZLDKNaRFXZ7G~xQM6nfDH3xxRBf3U42imJfmZVJMi1LwsUX4LDesL25jMQjKMXyrPB-HIVswjATk4hgWz3qwko1W6arxoICoAsFzb0LOT0TOyycgGN8Y1rsoGZVqMAZnKo75TpA1u1B-9l7Ap9KX96IhBZrpoTbHLbXDjv25bVZe4WyRR3~tGPUhQrGYqvejP4ZoJViQ0efNS-ku5GEMTz5uFIt6vRKQTjTqMZ24zFa6-AWNPfKOOAnbhKf6eHaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)