Abstract
Venous thromboembolism (VTE) is rare in healthy children, but is an increasing problem in children with underlying medical conditions. Pediatric VTE encompasses a highly heterogenous population, with variation in age, thrombosis location, and underlying medical comorbidities. Evidence from pediatric clinical trials to guide treatment of VTE is lacking so treatment is often extrapolated from adult trials and expert consensus opinion. Aspects unique to children include developmental hemostasis and the major role of central venous access devices. There is an absence of information regarding the optimal target levels of anticoagulation for neonates and infants and lack of suitable drug formulations. Anticoagulants, primarily low-molecular-weight heparin and warfarin, are used to treat children with symptomatic VTE. These drugs have significant limitations, including the need for subcutaneous injections and frequent monitoring. Randomized clinical trials of direct oral anticoagulants in pediatric VTE are ongoing, with results anticipated soon. These trials will provide new evidence and options for therapy that have the potential to improve care. International collaborative registries offer the ability to study outcomes of rare subgroups of pediatric VTE (eg, renal vein thrombosis), and will be important to ultimately guide therapy in a more disease-specific manner.
Introduction
Managing infants and children with venous thromboembolism (VTE) has become a daily activity of pediatric hematologists working in tertiary care hospitals. Treatment decisions remain challenging because of the lack of high-quality pediatric evidence and are often extrapolated from adult studies and/or based on expert consensus opinion.1 In 2018, the American Society of Hematology (ASH) published guidelines for the treatment of pediatric VTE; yet, most recommendations are based on very little evidence.2 Many knowledge gaps remain, particularly regarding the natural history and outcomes of VTE based on location and underlying patient population.
This manuscript will discuss the ASH treatment guidelines and highlight common clinical scenarios in which there is significant practice variation, including infants with VTE, duration of therapy for central venous access device (CVAD)-related thrombosis, asymptomatic thrombosis, thrombolysis, and use of direct oral anticoagulants (DOACs) in adolescents. Anticoagulants used in pediatric VTE will be reviewed, focusing on aspects unique to neonates and children.
Epidemiology
Overall, VTE is a rare event in children (1 in 100 000) compared with adults (1 in 1000).3-5 Conversely, VTE is an increasing problem in hospitalized children, from 5.3 events per 10 000 pediatric hospital admissions in the early 1990s to the current 30 to 58 events per 10 000 pediatric hospital admissions.5-8 Factors driving this increase include heightened awareness and more invasive technologies in children with underlying medical conditions.7 Harms from VTE include death, pulmonary embolism (PE), paradoxical emboli and stroke, organ dysfunction, infection, postthrombotic syndrome (PTS), loss of venous access, and pain. Hospital-acquired VTE in pediatric patients is associated with an increased hospital stay and cost.9 The estimated mortality rate associated with pediatric VTE is 2.2%, although this is likely an underestimate.10
The age distribution of pediatric VTE is bimodal, revealing peaks in neonates and adolescents.7 The early peak is mainly from CVADs, which are necessary to provide life-sustaining therapy to premature and critically ill infants, whereas the rise during adolescence generally reflects the development of adult VTE risk factors.5,8
The etiology of pediatric VTE is multifactorial, including both genetic and acquired risk factors. Greater than 90% of pediatric patients with VTE have multiple prothrombotic risk factors.4,10 The presence of a CVAD often leads to a perfect triad for thrombosis: endothelial injury from CVAD placement, venous stasis from disruption of venous flow, and often an underlying hypercoagulable state as the indication for the CVAD (eg, infection, cancer, congenital heart disease, inflammation). There is variation in VTE risk by CVAD type, with peripherally inserted central catheters having a higher risk than tunneled catheters.11,12
Pediatric VTE occur in a wide range of locations, including upper and lower deep veins, abdominal veins (inferior vena cava, renal, portal, or hepatic veins), right atrium, pulmonary arteries, and cerebral sinuses. Given the high prevalence of CVAD-associated VTE (CVAD-VTE), the location of the device is often responsible for the location of the thrombus.
Treatment
Treatment options for VTE include observation, anticoagulation, or thrombectomy (pharmacologic, pharmaco-mechanical, or surgical). When weighing options, risk of complications from the thrombus need to be balanced against risk of treatment, taking into consideration the patient’s underlying hemostatic system and comorbidities. Unfortunately, evidence to accurately assess the likelihood of these outcomes is lacking, so providers often use consensus-based guidelines as well as experience from prior cases or from adult studies to guide therapy. Whenever possible, treatment should be guided by a pediatric hematologist with expertise in thrombosis, either directly or in consultation.
The 2018 ASH Guidelines for treatment of pediatric VTE addressed a series of clinical questions using the McMaster University Grading of Recommendations Assessment, Development, and Evaluation approach.2 Recommendations were either strong or conditional, and stated the level of certainty based on the evidence using the rigorous Grading of Recommendations Assessment, Development, and Evaluation methodology. Of the 30 treatment recommendations, 26 were conditional and all 30 were based on “very low certainty of the evidence.”
These guidelines are a valuable resource and include detailed Evidence to Decision tables for each question based on systematic literature review. The Evidence to Decision tables address the effects of interventions, relative importance of clinical outcomes, feasibility, and acceptability of the intervention and resource utilization.2 Table 1 summarizes many of the treatment recommendations from these guidelines.
Summary of 2018 ASH treatment recommendations for acute pediatric VTE2
| VTE description . | Treatment recommendation . | Comments . |
|---|---|---|
| Symptomatic DVT or PE | • Anticoagulation | Observation may be necessary or reasonable for premature neonates or critically ill children at high risk of bleeding |
| Provoked VTE: treat ≤3 mo (if provoking factor is resolved) | ||
| Unprovoked VTE: treat 6-12 mo; consider longer duration based on patient’s preferences | ||
| • Avoid thrombolysis (unless life- or limb-threatening) | ||
| • Avoid IVC filter (unless absolute contraindication to anticoagulation) | ||
| Asymptomatic DVT or PE | • Anticoagulation or observation | Natural history is not well known; decision is likely to vary based on thrombus location and patient |
| Massive PE with hemodynamic compromise | • Thrombolysis followed by anticoagulation | |
| Submassive PE (no hemodynamic instability) | • Anticoagulation alone | |
| CVAD thrombosis | • See Figure 1 | |
| RVT | • Unilateral: anticoagulation alone | |
| • Bilateral: consider thrombolysis for bilateral RVT (life-threatening) | ||
| Portal vein thrombosis | • Occlusive: anticoagulation | |
| • Nonocclusive: observation (close radiologic follow-up) | ||
| Cerebral sinovenous thrombosis | • Anticoagulation | Decision in patients with intracranial hemorrhage needs to be individualized, but some patients may benefit from anticoagulation |
| VTE description . | Treatment recommendation . | Comments . |
|---|---|---|
| Symptomatic DVT or PE | • Anticoagulation | Observation may be necessary or reasonable for premature neonates or critically ill children at high risk of bleeding |
| Provoked VTE: treat ≤3 mo (if provoking factor is resolved) | ||
| Unprovoked VTE: treat 6-12 mo; consider longer duration based on patient’s preferences | ||
| • Avoid thrombolysis (unless life- or limb-threatening) | ||
| • Avoid IVC filter (unless absolute contraindication to anticoagulation) | ||
| Asymptomatic DVT or PE | • Anticoagulation or observation | Natural history is not well known; decision is likely to vary based on thrombus location and patient |
| Massive PE with hemodynamic compromise | • Thrombolysis followed by anticoagulation | |
| Submassive PE (no hemodynamic instability) | • Anticoagulation alone | |
| CVAD thrombosis | • See Figure 1 | |
| RVT | • Unilateral: anticoagulation alone | |
| • Bilateral: consider thrombolysis for bilateral RVT (life-threatening) | ||
| Portal vein thrombosis | • Occlusive: anticoagulation | |
| • Nonocclusive: observation (close radiologic follow-up) | ||
| Cerebral sinovenous thrombosis | • Anticoagulation | Decision in patients with intracranial hemorrhage needs to be individualized, but some patients may benefit from anticoagulation |
DVT, deep vein thrombosis; IVC, inferior vena cava; RVT, renal vein thrombosis.
Children with VTE are highly heterogenous and, when approaching treatment decisions, it can be helpful to consider highly prevalent subgroups (eg, neonates/infants, CVAD-related VTE, adolescents). Factors unique to children must be considered when making treatment decisions, including developmental hemostasis, limited vascular access and the role of CVADs, dietary differences, and the high prevalence of coexisting critical illness.
Anticoagulation
Anticoagulation is generally instituted in children with symptomatic VTE unless the patient is bleeding or at high risk for bleeding. The purpose of anticoagulation is to prevent clot extension, embolism, and recurrence. Challenges of anticoagulation in children include limited data, lack of pediatric formulations, need for dosing alteration secondary to developmental hemostasis, differences in pharmacokinetics, and lack of venous access to easily administer and monitor anticoagulant effect.
Recognizing that anticoagulants are high-risk medications, The Joint Commission has played an important role ensuring accredited US hospitals institute several performance elements as part of a National Patient Safety Goal to help reduce the likelihood of harm.13 These elements, recently updated, include having a defined anticoagulant management program as well as written protocols for the initiation and maintenance of anticoagulation (including baseline laboratory studies, drug doses, monitoring, and dose adjustments), and for the reversal of anticoagulation and management of bleeding.13 Hospitals must provide education about anticoagulation therapy to providers as well as patients and families, and institute a system to monitor and evaluate their anticoagulation safety practices. The anticoagulation National Patient Safety Goal was developed mainly for adult patients, but was also required and instituted across US children’s hospitals. Most pediatric centers developed written anticoagulation protocols based on published pediatric guidelines, modifying them as needed.1 Implementing elements of the National Patient Safety Goal increased pharmacy input and oversight at many centers, and along with ensuring a more consistent approach for dosing and monitoring, has likely improved the safe use of anticoagulants.
Before initiating anticoagulation, laboratory studies (complete blood count, prothrombin time, and activated partial thromboplastin time [aPTT]) should be obtained to assess the patient’s underlying coagulation status and risk of bleeding. If using low-molecular-weight heparin (LMWH), serum creatinine is important to assess renal clearance. Patients with impaired renal function may need increased monitoring, dose reduction, or an alternative drug. For preterm infants, a baseline head ultrasound to assess for intracranial hemorrhage is prudent. In addition, obtain a thorough clinical history of potential sites of bleeding (ie, recent surgery, gastrointestinal bleeding, trauma) when weighing the risks and benefits of anticoagulation.
Table 2 lists the common anticoagulants used in pediatrics, mechanism of action, pharmacokinetic properties, dosing, and therapeutic monitoring. Each of these drugs requires monitoring.1 Optimal target ranges for pediatric patients have not been established, so adult ranges are used, despite the limitations of this approach. Dalteparin, a LMWH, was recently approved by the Food and Drug Administration (FDA) for use in pediatric patients >1 month of age, and is the first anticoagulant to receive FDA approval for pediatric VTE. Enoxaparin, also a LMWH, is the most frequently used anticoagulant in pediatrics.7 LMWH is generally preferred over unfractionated heparin (UFH) because it is administered via subcutaneous injection, has limited drug interactions, and is easier to achieve therapeutic levels. UFH is usually reserved for patients at highest risk of bleeding or in renal failure because of its short half-life, reversibility (with protamine), and nonrenal clearance. Both LMWH and UHF require antithrombin (AT), which is lower in neonates and results in the need for age-specific dosing (Table 2). Enoxaparin is available in a multidose vial or prefilled syringes. The use of the multidose vial allows for exact dose titration and utilization of much smaller insulin needles for administration (31 gauge, 5/16-inch insulin syringe vs 27-gauge, 1/2-inch length prefilled syringe). An insulin syringe can be used because 1 unit on an insulin syringe is equivalent to 1 mg of enoxaparin.15 Whole milligram dosing of enoxaparin, rather than decimal dosing, appears to be safe and effective in children.16
Common anticoagulant therapies for use in pediatric VTE1,14,15
| Drug name . | Mechanism of action . | Pharmacokinetic properties and dosing . | Therapeutic monitoring (based on adult ranges) . | |
|---|---|---|---|---|
| UFH | Binds to AT and potentiates anticoagulant activity. The heparin-AT complex inactivates factors IIa (thrombin), Xa, XIa, and XIIa. | Half-life 0.5-2.5 h | Target range: aPTT: 1.5-2.5 times control OR UFH anti-Xa level: 0.3-0.7 U/mL | |
| Route: Continuous infusion | ||||
| Initial dose: | ||||
| Age <12 mo: bolus 75 U/kg followed by 28 U/kg/h | ||||
| Age >1-<12 y: bolus 75 U/kg followed by 20 U/kg/h | ||||
| Age >12 y: bolus 80 U/kg followed by 18 U/kg/h | ||||
| Enoxaparin (LMWH) | Binds to AT and potentiates anticoagulant activity. Has a reduced inhibitory activity against factor IIa (thrombin) relative to factor Xa. | Half-life 3-6 h, renal clearance | Target range: Enoxaparin anti-Xa peak: 0.5-1 U/mL (drawn 3-4 h after third dose) | |
| Route: Subcutaneous injection | ||||
| Initial dose: | ||||
| Age <2 mo: 1.5-1.7 mg/kg q12 h | ||||
| Age >2 mo: 1 mg/kg q12 h | ||||
| Dalteparin (LMWH) | Similar to enoxaparin. | Half-life 3-6 h, renal clearance | Target range: Dalteparin anti-Xa peak: 0.5-1 U/mL (drawn 3-4 h after third dose) | |
| Route: Subcutaneous injection | ||||
| Initial dose: | ||||
| Age 1 mo-<2 y: 150 IU/kg q12 h | ||||
| Age 2-<8 y: 125 IU/kg q12 h | ||||
| Age 8-<17 y: 100 IU/kg q12 h | ||||
| Fondaparinux | Synthetic pentasaccharide that binds AT and enhances inactivation of factor Xa. No inhibitory activity against factor IIa. | Half-life 17 h | Target range: Fondaparinux anti-Xa 0.5-1 mg/L (drawn 3-4 h after third dose) | |
| Route: Subcutaneous injection | ||||
| Initial dose: | ||||
| Age >1 y: 0.1 mg/kg q24 h | ||||
| Warfarin | Interferes with the cyclic conversion of vitamin K through the inhibition of vitamin K epoxide reductase. Resultant decrease in the posttranslational γ-carboxylation of vitamin K–dependent clotting factors II, VII, IX, and X and anticoagulants protein C and S. | Half-life 20-60 h | Target range: INR: 2-3 | |
| Route: Oral | ||||
| Loading dose: 0.2 mg/kg × 1 (if INR <1.3) (maximum, 10 mg) | ||||
| Check INR daily (days 2-4) and if the INR is: | ||||
| 1.1-1.3 | Repeat loading dose | |||
| 1.4-1.9 | 50% of loading dose | |||
| 2.0-3.0 | 50% of loading dose | |||
| 3.1-3.5 | 25% of loading dose | |||
| >3.5 | Hold until INR <3.5, restart at 50% loading dose | |||
| Drug name . | Mechanism of action . | Pharmacokinetic properties and dosing . | Therapeutic monitoring (based on adult ranges) . | |
|---|---|---|---|---|
| UFH | Binds to AT and potentiates anticoagulant activity. The heparin-AT complex inactivates factors IIa (thrombin), Xa, XIa, and XIIa. | Half-life 0.5-2.5 h | Target range: aPTT: 1.5-2.5 times control OR UFH anti-Xa level: 0.3-0.7 U/mL | |
| Route: Continuous infusion | ||||
| Initial dose: | ||||
| Age <12 mo: bolus 75 U/kg followed by 28 U/kg/h | ||||
| Age >1-<12 y: bolus 75 U/kg followed by 20 U/kg/h | ||||
| Age >12 y: bolus 80 U/kg followed by 18 U/kg/h | ||||
| Enoxaparin (LMWH) | Binds to AT and potentiates anticoagulant activity. Has a reduced inhibitory activity against factor IIa (thrombin) relative to factor Xa. | Half-life 3-6 h, renal clearance | Target range: Enoxaparin anti-Xa peak: 0.5-1 U/mL (drawn 3-4 h after third dose) | |
| Route: Subcutaneous injection | ||||
| Initial dose: | ||||
| Age <2 mo: 1.5-1.7 mg/kg q12 h | ||||
| Age >2 mo: 1 mg/kg q12 h | ||||
| Dalteparin (LMWH) | Similar to enoxaparin. | Half-life 3-6 h, renal clearance | Target range: Dalteparin anti-Xa peak: 0.5-1 U/mL (drawn 3-4 h after third dose) | |
| Route: Subcutaneous injection | ||||
| Initial dose: | ||||
| Age 1 mo-<2 y: 150 IU/kg q12 h | ||||
| Age 2-<8 y: 125 IU/kg q12 h | ||||
| Age 8-<17 y: 100 IU/kg q12 h | ||||
| Fondaparinux | Synthetic pentasaccharide that binds AT and enhances inactivation of factor Xa. No inhibitory activity against factor IIa. | Half-life 17 h | Target range: Fondaparinux anti-Xa 0.5-1 mg/L (drawn 3-4 h after third dose) | |
| Route: Subcutaneous injection | ||||
| Initial dose: | ||||
| Age >1 y: 0.1 mg/kg q24 h | ||||
| Warfarin | Interferes with the cyclic conversion of vitamin K through the inhibition of vitamin K epoxide reductase. Resultant decrease in the posttranslational γ-carboxylation of vitamin K–dependent clotting factors II, VII, IX, and X and anticoagulants protein C and S. | Half-life 20-60 h | Target range: INR: 2-3 | |
| Route: Oral | ||||
| Loading dose: 0.2 mg/kg × 1 (if INR <1.3) (maximum, 10 mg) | ||||
| Check INR daily (days 2-4) and if the INR is: | ||||
| 1.1-1.3 | Repeat loading dose | |||
| 1.4-1.9 | 50% of loading dose | |||
| 2.0-3.0 | 50% of loading dose | |||
| 3.1-3.5 | 25% of loading dose | |||
| >3.5 | Hold until INR <3.5, restart at 50% loading dose | |||
q, every.
Warfarin remains the mainstay for oral anticoagulation in pediatric patients. In addition to the known challenges with warfarin, unique pediatric concerns include developmental changes in vitamin K–dependent proteins, age-related dietary differences (ie, high vitamin K in formula), and no liquid formulation. Warfarin dosing studies in children have demonstrated that age is the greatest determining factor, and infants require the highest per kilogram dose.17,18 International normalized ratio (INR) monitoring is extrapolated from adult studies despite the fact that at the same INR level children’s plasma thrombin generation is delayed and decreased by 25% compared with adults.19 Because of a lack of liquid formulation and without data to support stability, warfarin pills are commonly crushed and dissolved in water. When warfarin is administered via a gastronomy or jejunal tube, there can be challenges. Warfarin is absorbed in the stomach, so continuous feeds can significantly affect absorption and dosing. In the setting of continuous gastronomy tube feeds, it is recommended to hold feeds 1 hour before and after warfarin administration.20,21 If converting from jejunal to gastric feeds, a dose reduction may be necessary and at the very least should prompt close INR monitoring.
Although DOACs are dominating the landscape for VTE in adults, pediatric trials, under way since 2010, are ongoing.22 DOACs approved for adult VTE include the direct factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) and direct thrombin inhibitor, dabigatran. Adult guidelines for treatment of VTE now recommend DOACs over warfarin.2,23 The 2018 ASH guidelines for pediatric VTE recommend that DOACs not be used in children until completion of clinical trials.2 For the first time in pediatric thrombosis, there are several pediatric investigational programs for anticoagulants.24 These trials will provide essential information regarding the pharmacokinetics, pharmacodynamics, pediatric formulations, safety, and efficacy of these drugs in children.
Given the benefits of DOACs compared with warfarin or enoxaparin (lack of interaction with diet, fewer drug interactions, no need for monitoring), it is not surprising that they are being prescribed with increasing frequency to adolescent patients with VTE.25 Rationale for considering off-label use in adolescent patients includes recognizing that hemostasis in these patients is similar to adults and the advantages of DOACs are expected to apply to adolescents. There is increasing data from phase 1 pharmacokinetic and phase 2 studies, as well growing provider experience from participating in clinical trials and local select use.24 Acknowledging the lack of published pediatric data, practical issues when considering off-label use of DOACs in select adolescents are listed in Table 3.26-28
Practical considerations for using off-label use of DOACS in adolescents
| Guidance . | Comments . |
|---|---|
| Document informed discussion with patient and family regarding alternatives and off-label use | |
| Restrict to adult dosing recommendations (until approved for children) | Only a minority (∼10%) of patients in adult clinical trials were low weight (50-60 kg) |
| Weight >50 kg | |
| Restrict to patients with good renal function | |
| CrCl >60 mL/min | All of the DOACs are renally excreted |
| Guidance in package inserts regarding dose reduction for moderate kidney disease | Rates of bleeding are higher in adults with chronic renal disease |
| Do not use in patients with antiphospholipid antibody syndrome | |
| Higher rate of thrombotic events25 | DOACs interfere with the lupus anticoagulant assay (false-positive) |
| Gastrointestinal considerations | DOACs are absorbed in stomach and proximal intestine |
| No food interactions | |
| Rivaroxaban and dabigatran should be taken with meals | |
| Drug interactions | Good practice to check with pharmacy specialist if patients is on multiple medications |
| Very few drug interactions | |
| Avoid DOACs for patients on drugs that interact, including: amiodarone, azole antifungals, clarithromycin, rifampin, phenytoin, carbamazepine | |
| Heavy menstrual bleeding | Strategies to address HMB in women on anticoagulation are addressed in Boonyawat et al.26 |
| Heavy menstrual bleeding may be more common with direct Xa inhibitors than with warfarin | |
| Education/follow-up/communication | Switching a patient who is nonadherent to warfarin to a DOAC will not solve the problem |
| Although drug monitoring is not necessary, close follow-up improves adherence | |
| Hold for elective procedures59 | |
| Low bleeding risk procedure, normal renal function: 24 h | |
| High bleeding risk procedure, normal renal function: 48-72 h | |
| Reversal for life-threatening bleeding or urgent surgery | Limited data on efficacy27,28 |
| Idarucizumab: reversal agent for dabigatran | |
| Andexanet α: reversal agent for factor Xa inhibitors |
| Guidance . | Comments . |
|---|---|
| Document informed discussion with patient and family regarding alternatives and off-label use | |
| Restrict to adult dosing recommendations (until approved for children) | Only a minority (∼10%) of patients in adult clinical trials were low weight (50-60 kg) |
| Weight >50 kg | |
| Restrict to patients with good renal function | |
| CrCl >60 mL/min | All of the DOACs are renally excreted |
| Guidance in package inserts regarding dose reduction for moderate kidney disease | Rates of bleeding are higher in adults with chronic renal disease |
| Do not use in patients with antiphospholipid antibody syndrome | |
| Higher rate of thrombotic events25 | DOACs interfere with the lupus anticoagulant assay (false-positive) |
| Gastrointestinal considerations | DOACs are absorbed in stomach and proximal intestine |
| No food interactions | |
| Rivaroxaban and dabigatran should be taken with meals | |
| Drug interactions | Good practice to check with pharmacy specialist if patients is on multiple medications |
| Very few drug interactions | |
| Avoid DOACs for patients on drugs that interact, including: amiodarone, azole antifungals, clarithromycin, rifampin, phenytoin, carbamazepine | |
| Heavy menstrual bleeding | Strategies to address HMB in women on anticoagulation are addressed in Boonyawat et al.26 |
| Heavy menstrual bleeding may be more common with direct Xa inhibitors than with warfarin | |
| Education/follow-up/communication | Switching a patient who is nonadherent to warfarin to a DOAC will not solve the problem |
| Although drug monitoring is not necessary, close follow-up improves adherence | |
| Hold for elective procedures59 | |
| Low bleeding risk procedure, normal renal function: 24 h | |
| High bleeding risk procedure, normal renal function: 48-72 h | |
| Reversal for life-threatening bleeding or urgent surgery | Limited data on efficacy27,28 |
| Idarucizumab: reversal agent for dabigatran | |
| Andexanet α: reversal agent for factor Xa inhibitors |
CrCl, creatinine clearance.
Although it may be reasonable to begin to extrapolate adult DOAC experience to relatively healthy adolescents with VTE, it is essential to wait for clinical trial data to help guide dosing in younger children and to better understand the effect of these drugs for indications such as congenital heart disease and CVAD-related thrombosis. The phase 3 pediatric trials were not designed to independently demonstrate safety or efficacy, so postapproval studies will be important. Furthermore, because a large proportion of children with VTE has complex medical conditions, and is often hospitalized with variable enteral intake, many of these patients will not be good candidates for DOACs, and monitoring may be necessary in certain subsets of patients.
Thrombolysis
Anticoagulation is generally effective in managing VTE, but there are times when more rapid clot resolution may be necessary. In these situations, pharmacologic thrombolysis may be of benefit. An increased risk of major bleeding, particularly in neonates, is the primary drawback, so this therapy is generally reserved for limb-, life-, or organ-threatening events.1 VTE that fits these criteria is rare, but include bilateral renal vein thrombosis, superior vena cava syndrome, cerebral sinovenous thrombosis with neurologic decline, “phlegmasi alba dolens” (extremity deep vein thrombosis [DVT] with pending limb ischemia), intracardiac thrombi causing cardiovascular instability, and massive pulmonary embolism.
Although there are several thrombolytic agents available, none have been studied in children. The drug used most in pediatrics is alteplase, a recombinant tissue plasminogen activator (rtPA).29 Alteplase has a short half-life (3-5 minutes) and can be administered systemically or at the site of thrombosis. Dosing regimens of rtPA for systemic thrombolysis vary widely.30 In general, published reports include “low-dose” infusions (0.01-0.06 mg/kg/h) for 6 to 72 hours or “high-dose” infusion (0.1-0.6 mg/kg/h) for 2 to 6 hours, repeated if needed.29 Use of adjuvant UFH during systemic thrombolysis also varies.30 Although there is no “therapeutic range” for thrombolysis, laboratory monitoring (complete blood count, prothrombin time, partial thromboplastin time, fibrinogen, D-dimer) every 6 to 12 hours during systemic thrombolysis is recommended to assess for risk of bleeding and thrombolytic response.29 In neonates, baseline and follow-up head ultrasound should be performed to assess for intracranial hemorrhage. Patients should be monitored closely for clinical improvement as well as for bleeding in an intensive care unit, with a multidisciplinary team that includes the intensivist, hematologist, and radiologist. Site-directed endovascular rtPA requires an experienced pediatric interventionalist (radiologist or cardiologist), but may be preferred over systemic rtPA when feasible because it may shorten the duration of therapy and therefore reduce the risk of bleeding.
Although the ASH pediatric guidelines recommend against routine thrombolysis for VTE, the guideline panel acknowledged that there may be individuals that would benefit.2 Catheter-directed thrombolysis did not improve rates of PTS in a randomized trial of adults with DVT involving the iliac or common femoral veins.31 However, in a subset of patients with iliofemoral DVT, there was a significant reduction in the severity of PTS.32 Therefore, thrombolysis may be reasonable to consider in an otherwise healthy adolescent with occlusive iliofemoral vein thrombosis and severe symptoms in attempt to reduce the risk of severity of PTS, which occurs in nearly 30% of patients.33 In addition, patients with severe thrombotic symptoms related to anatomic risk factors for thrombosis may benefit more aggressive therapy.
Bleeding complications
Bleeding is the most relevant risk of anticoagulation, and pediatric data are limited and affected by confounding by indication. Reported bleeding rates for UFH range from 1.5% to 24%.1 This wide range likely reflects the variation in the patient population studied as well as the fact that UFH is commonly reserved for use in the highest risk patients. LMWH has reported bleeding rates ranging from 0.8% to 5.6%.1 Reported major bleeding rates with warfarin are variable based on indication and study type, with a range of 0.5% to 12.2%.34-36 Antiplatelet medications should be avoided secondary to an increased risk of bleeding. For menstruating females, attention should be paid to the development of abnormal uterine bleeding.
Protamine is the only reversal agent for UFH and LMWH. UFH has such a short half-life that turning off the infusion is sufficient to treat many forms of bleeding. If immediate reversal is required, protamine can be used. The duration of protamine is approximately 2 hours, and a heparin rebound effect may occur. Protamine only partially reverses the anticoagulation effect of LMWH, and a repeat dose, based on anti-Xa levels, may be required secondary to the long half-life of LMWH.37
The antidote for warfarin is vitamin K, although there is a delay of hours before a hemostatic effect is seen. In the setting of life-threatening bleeding, vitamin K replacement should be augmented with the use of a 4-factor prothrombin complex concentrate (PCC) or fresh frozen plasma.1 A 4-factor PCC is a plasma-derived factor concentrate that contains the vitamin K–dependent coagulation factors II, VII, IX, and X and anticoagulant proteins C and S. Kcentra (PCC [human], CSL Behring, Kankakee, IL), is approved by the FDA for the urgent reversal of warfarin. The benefit to using a PCC over fresh frozen plasma is the smaller volume and higher concentrations of relevant procoagulant and anticoagulant proteins.
Targeted reversal agents for the DOACs have been developed and are available for patients with life-threatening or uncontrolled bleeding, although data regarding the efficacy of these parenteral drugs are limited.27,28 Idarucizumab is a monoclonal antibody that binds to and neutralizes dabigatran.27 Andexanet α is a modified inactive form of factor Xa that binds to and sequesters Xa inhibitors.28
Physical activity and anticoagulation
Children receiving anticoagulants should be counseled regarding appropriate activities. Toddlers may benefit from a soft helmet to prevent facial or scalp bruising with falls. In general, guidelines are given to “keep 1 foot on the ground at all times” to minimize falls from a height and head trauma. Providing guidance regarding sports participation for older children on anticoagulation is a challenge. Although sports participation has clear health and psychosocial benefits, there is risk. The consensus has been to restrict participation in collision or contact sports while on anticoagulation because of the high risk of injury and resultant bleeding.38,39 Providers should be aware that the removal of participation in a high-risk sport can be a significant psychosocial stressor; this should be addressed at clinic visits. Recognizing the importance of continued play to elite athletes, some have proposed an intermittent dose strategy for adults on DOACs.40 Patients returning to noncontact sports should have a structured and gradual return after an acute thrombotic event. It is reasonable to recommend a gradual return to a noncontact activity starting after 3 weeks (highest risk for embolization) with full participation as soon as 6 weeks, with the caveat that any activity that precipitates return of symptoms should be discontinued.39
Duration of therapy
Once anticoagulation has been initiated, questions arise regarding the duration of therapy. Pediatric guidelines recommend treating provoked VTE for a maximal duration of 3 months.2 Treating CVAD-VTE for a shorter duration (generally 6 weeks) has become common in many centers, despite little evidence.41 Importantly, there is an ongoing multicenter, randomized clinical trial evaluating 6 weeks compared with 3 months of anticoagulation in patients <21 years of age with a provoked VTE.42 This trial, Kids-DOTT, with a target sample size of 800 patients and projected completion date of 2021, will provide high-quality evidence for duration of therapy for pediatric provoked VTE and has the potential to greatly affect the standard of care.42
Classifying VTE as provoked vs unprovoked is not always straightforward. Provoked VTE occurs in the setting of an acquired risk factor that may be transient (surgery, CVAD, infection, estrogen therapy) or persistent (active cancer, inflammatory bowel disease, congenital heart disease). Unprovoked VTE occur in the absence of an environmental or acquired risk factor.43
Children with VTE often have both transient and persistent risk factors (eg, inflammatory bowel disease and a CVAD). The thrombotic risk in some diseases, including cancer, nephrotic syndrome, and rheumatic/inflammatory disorders, changes over time with disease control. The duration of anticoagulation therapy in a patient with persistent acquired prothrombotic risk factors is generally made on an individual basis. Less than 10% of pediatric VTE are unprovoked, the majority occurring in adolescent patients. Recommended duration of therapy for unprovoked VTE in children is 6 to 12 months, recognizing that there may be additional clinical factors or patient preference that may alter this duration.2 Patients that warrant consideration of longer duration therapy include those with antiphospholipid antibody syndrome, life-threatening index VTE, strong/combined inherited thrombophilia, or recurrent unprovoked VTE.
Neonates/Infants
Children ≤1 year account for the largest annual proportion of pediatric VTE. This group includes high proportions of premature neonates and infants with congenital heart disease.5 Aspects distinct to this age group include developmental hemostasis, which affects not only response to anticoagulants but also bleeding risk, unique sites of thrombosis (eg, renal vein, portal vein, cerebral sinuses), and lack of pediatric-specific drug formulations.
Developmental hemostasis refers to the evolving hemostatic system in neonates.44,45 Although the system maintains hemostasis in healthy neonates, concentrations of many coagulation factors vary and change rapidly over the first 6 months of life. In general, neonates have lower concentrations of many procoagulant and anticoagulant proteins, placing them at greater risk of both bleeding and thrombotic complications.44 These differences are even more profound in premature neonates.46 Most relevant to treatment of VTE is reduced levels of AT, which, as previously discussed, is necessary for the anticoagulant action of both UFH and LMWH, explaining some of the age-related differences observed with these drugs. Most values normalize by 6 months of age, although changes are seen throughout childhood.
Patients in this age group are small and fragile, often have multiple coexisting conditions, and blood sampling is extremely difficult. These obstacles have impeded high-quality clinical research of VTE. A Cochrane review of heparin for the treatment of thrombosis in neonates concluded that there were no studies to recommend or refute the use of heparin for neonates with thrombosis.47 It is not known whether the adult anti-Xa target ranges for UFH or LWMH are necessary or appropriate to treat neonates with VTE. There is a greater discrepancy between aPTT and anti-Xa in neonates on UFH compared with adults, suggesting that this approach is problematic.48 This remains a significant knowledge gap and further investigation is greatly needed.
The true risk-to-benefit ratio of anticoagulation compared with no anticoagulation in neonates/infants with VTE is not known. Therefore, more in this age group than any other, treatment is carefully individualized recognizing these limitations. Close observation with follow-up imaging, rather than anticoagulation, is often a reasonable management option for a critically ill neonate or infant with VTE.
CVAD-associated VTE
A CVAD is the most common risk factor for thrombosis in children.5 There can be variable degrees of clot involvement with the central line itself and with the vein, ranging from obstruction of only the CVAD tip, to partial or full vein occlusion.49 This can result in a wide variation of clinical symptoms including asymptomatic to symptomatic with a nonfunctioning CVAD and a swollen and/or painful extremity.49
Whether to treat asymptomatic CVAD-associated thrombosis is debatable. Current ASH guidelines suggest either treating or not treating asymptomatic thrombosis.2 In a prospective cohort study of 189 critically ill children with jugular or femoral vein CVAD, 22% were identified to have asymptomatic thrombosis using screening ultrasound.50 No patient developed acute or long-term sequelae with 2 years of follow-up. The majority of patients in this cohort had congenital heart disease and the CVADs were of relatively short duration. Further studies are necessary to validate these findings but suggests that all asymptomatic CVAD-associated thrombosis might not require specific treatment, particularly if the CVAD is removed.
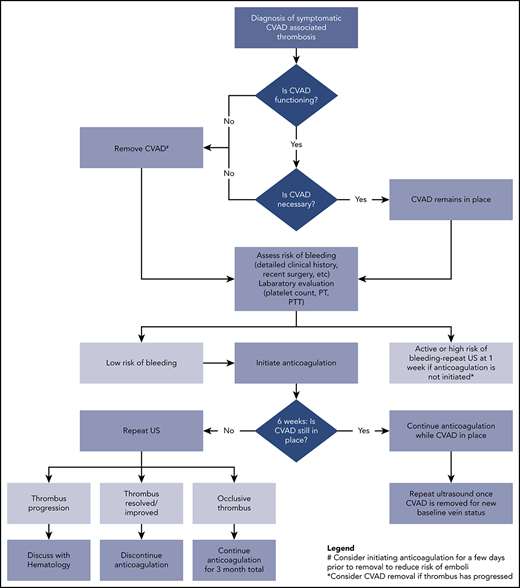
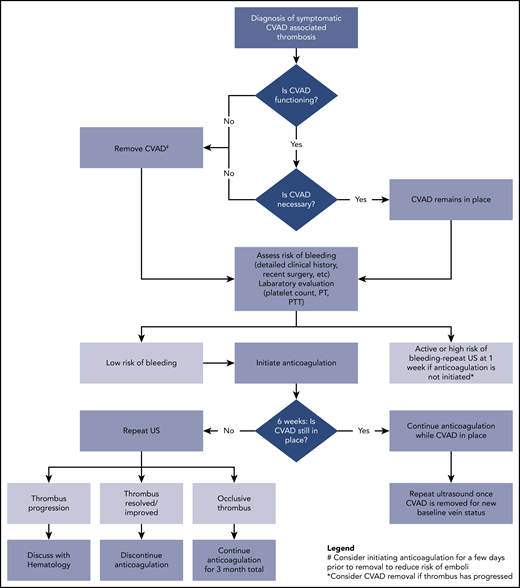
A treatment pathway for symptomatic CVAD-VTE is shown in Figure 1. Key decision points include whether to remove the device, the safety of initiating anticoagulation, and the duration of treatment. The ASH guidelines favor leaving the device in place if functional and clinically necessary over removal and placement of a new CVAD.2 If the line is nonfunctional or no longer needed clinically, then removal is recommended although because of a concern for embolization this should be done after a few days of anticoagulation.2,51,52 Last, a recent meta-analysis and guidelines do not support a role for thrombophilia testing in CVAD-associated thrombosis.53,54
Clinical pathway for management of acute CVAD-associated thrombosis in neonates and children. PT, prothrombin time; PTT, partial thromboplastin time; US, ultrasound
Clinical pathway for management of acute CVAD-associated thrombosis in neonates and children. PT, prothrombin time; PTT, partial thromboplastin time; US, ultrasound
Adolescents
Risk factors in adolescent VTE are different from younger children.55 They are more likely to present with unprovoked VTE, have the highest prevalence of inherited thrombophilia, and may have an underlying anatomic risk factor including thoracic outlet obstruction, May-Thurner syndrome (compression of the left common iliac vein by the right iliac artery), or inferior vena cava anomaly.56 Other common risk factors include malignancy, obesity, hormonal contraception, rheumatologic/inflammatory disorders, and immobility. In many respects, adolescents with VTE are similar to adults, and so applying evidence-based VTE treatment guidelines is generally sound practice. Nonetheless, there are some distinctions that should be considered. The risk of recurrence in adolescents appears to be higher compared with the overall estimate of recurrent VTE in children (22% vs 8%).10,55 Individuals with strong inherited thrombophilias often present as teenagers. Duration of anticoagulation therapy in a pediatric patient who presents with unprovoked VTE and a strong thrombophilia has not been established. The ASH guidelines recommend 6 to 12 months of therapy, recognizing the burden and absence of data to support longer duration.2 DOACs, which have the potential to be more tolerable than current options (warfarin or LMWH), may offer an alternative for extended duration therapy in select pediatric patients, similar to recommendations for adults with unprovoked VTE.23
Conclusions
Progress in advancing care of neonates and children with VTE has been slow.57 Improving knowledge of the natural history of different subtypes of thrombosis by age and location would enhance current thinking about harms and benefits of treatment more specific to the patient.57 This will require international registries, which are currently being developed.58 Results from the ongoing DOAC pediatric investigational programs will provide, for the first time, high-quality treatment and outcome data on hundreds of pediatric patients. Availability of safe and effective oral agents with pediatric data to support use would be of clear benefit. Although there will remain many knowledge gaps requiring further investigation, these studies will help advance the treatment of VTE in pediatrics.
Authorship
Contribution: C.W. and L.R. wrote, edited, and revised this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leslie Raffini, Pediatrics, Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania, 11022 Colkett Research Building, 3501 Civic Center Blvd, Philadelphia, PA 19018; e-mail: raffini@email.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal