In the report by Park et al,1 we learn that a cauldron of hypoxic marrow with vascular tortuosity and sinusoidal stasis is the pot from which hematopoietic output in sickle cell disease (SCD) is made. The marrow environment is a unique concoction of oxidative, vasoactive, and neoangiogenesis substances that are associated with collapsed, slow-flowing sinusoids clogged with erythroid and granulocytic aggregates (see figure). It is in this milieu that the hematopoietic niche with its cell-intrinsic (CXCL12-mesenchymal stem cells) and cell-extrinsic (HIF-1α, VEGF, sVCAM1, etc) features is altered.2 The perturbation prompts hematopoietic progenitor cells to proliferate, egress, and degrade to a certain extent the homeostasis that is critical in maintaining long-term repopulating cell potential. Ineffective erythropoiesis also is induced under oxidative stress and inflammation that characterize the sickle marrow. Remarkably, as shown in this report, these aberrant conditions are transferable upon transplantation of humanized sickle mouse bone marrow into healthy mouse recipients.
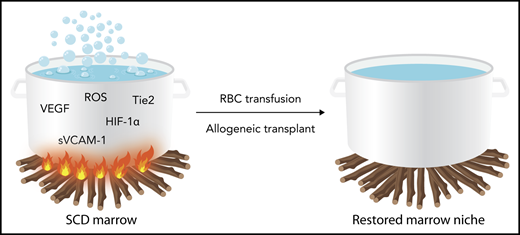
Schematic representation of the disordered bone marrow milieu in sickle cell disease. The inflammatory stimulus represented by fire under the pot is dampened by regular RBC transfusions or allogeneic bone marrow transplantation, which can correct the disordered milieu. HIF-1α, hypoxia inducing factor-1α; RBC, red blood cell; ROS, reactive oxygen species; sVCAM, soluble (circulating) vascular cell adhesion molecule; Tie2, tyrosine kinase with immunoglobulin-like and EGF-like domains 2; VEGF, vascular endothelial growth factor. Professional illustration by Somersault18:24.
Schematic representation of the disordered bone marrow milieu in sickle cell disease. The inflammatory stimulus represented by fire under the pot is dampened by regular RBC transfusions or allogeneic bone marrow transplantation, which can correct the disordered milieu. HIF-1α, hypoxia inducing factor-1α; RBC, red blood cell; ROS, reactive oxygen species; sVCAM, soluble (circulating) vascular cell adhesion molecule; Tie2, tyrosine kinase with immunoglobulin-like and EGF-like domains 2; VEGF, vascular endothelial growth factor. Professional illustration by Somersault18:24.
The cause and consequences of the sickle mutation are well known. The mutation exchanges the amino acid valine for glutamine at position 6 of the β-globin gene and creates a hydrophobic pocket in the hemoglobin molecule, which under low-oxygen tension promotes the formation of long sickle hemoglobin polymers.3 The propensity for this intracellular event causes its downstream pathogenic sequelae, including hemolysis, anemia, alterations in RBCs, and vascular endothelium and vascular tone. Together these changes promote vasoocclusion and tissue injury that affect virtually every organ in the body.4 It is perhaps not surprising that the marrow hematopoietic organ is also adversely affected by these same phenomena. It also stands to reason that therapeutic interventions that mitigate the sickle phenotype might also repair the disordered bone marrow.
This prediction was illustrated by results in Park et al. They observed that the tortuous, slow-flow, collapsed marrow sinusoids of the humanized sickle mice normalized after a 6-week blood transfusion period during which sickle hemoglobin levels declined precipitously. In addition, the elevated levels of soluble neoangiogenic and vasculopathic soluble factors were reversed and oxidative stress in erythroid progenitors also declined. A remarkably similar response was elicited by the transplantation of healthy murine donor marrow in the humanized sickle recipient mice.
These observations have compelling implications for clinical allogeneic hematopoietic cell and autologous gene-modified cell transplantation SCD. With regard to allogeneic transplantation, a critical barrier to a successful outcome is graft rejection and recurrent SCD, which occurs much more frequently in SCD than in hematological malignancies. The reasons for this are not entirely certain, but sensitization to histocompatibility antigens during pretransplant transfusion exposures and immunological rejection of the donor cells has been hypothesized.5 This report, however, suggests that the marrow niche itself might not be conducive to donor engraftment, perhaps exacerbated by an aggressive chemotherapy conditioning regimen. In autologous transplantation of gene-modified hematopoietic stem and progenitor cells, mobilization and collection of long-term repopulating cells are critical to a successful outcome. The possibility that mobilized hematopoietic cell products might be overrepresented by less-primitive progenitor populations that will not contribute to long-term hematopoiesis also comes to mind.
Of interest, the routine use of preventive exchange RBC transfusions administered before commencing treatment has been instituted in clinical transplantation protocols for SCD.6 The principal intention of the RBC transfusion is to reduce the risk of experiencing a clinical vasoocclusive event during or after the transplantation procedure. In addition, hematopoietic cell harvesting for autologous gene modifications therapies in SCD has shifted broadly from marrow harvesting to plerixafor-mobilization and peripheral blood cell harvesting.7-9 This procedure also is preceded by exchange RBC transfusion to reduce the risk of a clinical vasoocclusive event after the plerixafor-mediated leukocytosis that accompanies mobilization. It is serendipitous that another potential benefit of RBC transfusions before mobilization and transplantation as reported by Park et al is that transfusions might also favorably alter the sickle marrow milieu and improve hematopoietic progenitor cell harvesting and promote engraftment of allogeneic donor and autologous gene-modified hematopoietic cells.
This interesting report appears to directly address the difficult problem of graft rejection that has challenged allogeneic hematopoietic cell transplantation for SCD. It also suggests that in gene therapy for SCD, in which there is transfer of both modified and unmodified hematopoietic cells, the gene-modified cell product must contain a sufficient fraction of corrected hematopoietic stem cells to re-model the marrow niche and ensure production of erythroid cells without oxidative stress. In addition, the short-term institution of RBC transfusions to reduce the fraction of sickle RBCs several months in advance of stem cell harvesting and transplantation might be prudent.
Conflict-of-interest disclosure: M.C.W. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal