In this issue of Blood, Burroughs et al show impressive results in 129 pediatric patients who have undergone allogeneic hematopoietic stem cell transplantation (HCT) in North America between 2005 and 2015.1
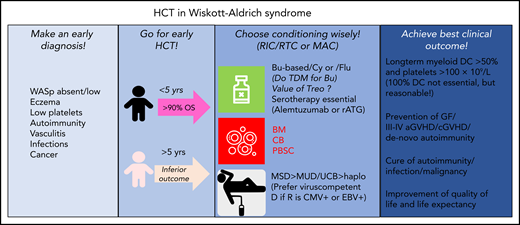
Diagnostic and therapeutic proposals for optimization of HCT in WAS. aGVHD, acute graft-versus-host-disease; BM, bone marrow; Bu, busulfan; CB, cord blood; cGVHD, chronic graft-versus-host-disease; D, donor; EBV, Epstein Barr virus; GF, graft failure; haplo, haploidentical; MAC, myeloablative conditioning; PBSC, peripheral blood stem cells; R, recipient; rATG, rabbit; RIC, reduced-intensity conditioning; RTC, reduced-toxicity conditioning; Treo, treosulfan; TDM, therapeutic drug monitoring; UCB, unrelated cord blood; WASp, Wiskott-Aldrich-Syndrome protein.
Diagnostic and therapeutic proposals for optimization of HCT in WAS. aGVHD, acute graft-versus-host-disease; BM, bone marrow; Bu, busulfan; CB, cord blood; cGVHD, chronic graft-versus-host-disease; D, donor; EBV, Epstein Barr virus; GF, graft failure; haplo, haploidentical; MAC, myeloablative conditioning; PBSC, peripheral blood stem cells; R, recipient; rATG, rabbit; RIC, reduced-intensity conditioning; RTC, reduced-toxicity conditioning; Treo, treosulfan; TDM, therapeutic drug monitoring; UCB, unrelated cord blood; WASp, Wiskott-Aldrich-Syndrome protein.
Wiskott-Aldrich syndrome (WAS) is an X-linked disorder caused by hemizygous mutations in the WAS gene leading to reduced/absent expression of the WAS protein, a major regulator of the actin cytoskeleton in hematopoietic cells. Affected boys experience severe microthrombocytopenia, eczema, and progressively deteriorating immune functions leading to autoimmunity, infections, and cancer. WAS is a life-threatening disease that can only be cured by allogeneic HCT1-4 or gene therapy.5
Infants and children aged <5 years benefited most from HCT, with a 5-year overall survival (OS) of 94%, whereas patients aged ≥5 years achieved poorer outcomes (66%). Myeloid donor chimerism (DC) >50% was associated with superior platelet production. Preexisting autoimmune disease, mainly autoimmune cytopenias, responded favorably to HCT. The preparative regimen consisted of mainly busulfan-based reduced intensity/toxicity conditioning or myeloablative conditioning regimens, including serotherapy. Excellent results were obtained with HLA-matched sibling donors (MSDs), 9/10-10/10 HLA–matched unrelated donors (MUDs), and unrelated cord blood transplants (UCBTs).
I remember in 2008 when one of my patients, at the age of 3 months, was diagnosed with WAS. Because of rare HLA alleles with no available MUD, at 2 years of age, the patient received a 7/10 (5/6) HLA–mismatched UCBT. He was negative for cytomegalovirus (CMV)/Epstein-Barr virus, and no virus-specific T cells were needed in his graft. The conditioning was myeloablative with busulfan/fludarabine. Therapeutic drug monitoring of busulfan was performed and resulted in a 50% dose reduction. Serotherapy with rabbit antithymocyte globulin (thymoglobulin; 4 × 2.5 mg/kg) was given to reduce the risks of graft-versus-host disease (GVHD). The patient achieved engraftment, and the HCT was uneventful. At latest follow-up in 2019, he exhibited 100% DC, normalized humoral immunity, and thymic function. Growth and length (both 75th-90th percentile), platelets (227 × 109/L), and results of lung function tests were all normal, and there was no chronic GVHD. This was a satisfactory clinical outcome.
Burroughs et al show on a much larger scale that UCBT (30% of their reported transplant cases) has become a good alternative to MSD/MUD transplants in WAS and achieved a 5-year OS of 90%. Very young children and infants who are less likely infected with viruses such as CMV and with less disease burden can benefit from timely UCBT. Previous reports of UCBT in 90 patients reported inferior results, reaching OS rates of 75% mainly due to infectious deaths.4 The other finding of Burroughs et al that patients aged ≥5 years had poorer outcomes is not new. It is noteworthy, however, that the group of patients aged ≥5 years consisted of only 12 patients (9%), whereas 117 patients (91%) were <5 years of age at HCT. Statisticians do not favor age thresholds in medicine, but clinicians love them because they are helpful to facilitate therapeutic decisions.
It is far more interesting to understand why very young patients with WAS do better after HCT. WAS is undisputedly a progressive disease of the immune system, and risk scores as well as disease burden clearly increase with age.2,3 But what are the reasons to wait with HCT until the patient becomes ≥5 years of age? A milder clinical course or nonavailability of suitable donors? What were the reasons that patients ≥5 years of age died after HCT? Previous splenectomy did not play a prognostic role. In the report of Burroughs et al, transplant-related mortality occurred mainly during the first year due to GHVD, infections, hemorrhage, and multiorgan failure, but the data are currently insufficient to answer these questions. Their rationale to perform transplants in children early and independent of their WAS scores is nevertheless convincing.
Moratto et al3 reported 5-year OS/transplant-related mortality/graft failure rates after HCT for WAS of 82%/18%/5%, respectively. In the study by Burroughs et al, these rates improved to 91%/9%/5%, although autoimmunity, declining DC, graft failure, grade 3 to 4 acute GVHD, and chronic GVHD remained serious complications after HCT.
Mixed DC in whole blood, T cells, and B cells were not directly associated with an increased incidence of de novo autoimmunity, which was an unexpected finding. In a prior article by Ozsahin et al,2 de novo autoimmunity developed in 20% (19 of 96 patients) post-HCT and was associated with mixed DC (6 had full DC, and 13 had mixed or split DC). They occurred more frequently in MUD (n = 9) but less in MSD (n = 5) and haploidentical (n = 5) transplants. In the cohort of Burroughs et al, MUD still had the highest numbers (23%) of de novo autoimmunity, this time independent of the degree of DC, whereas autoimmunity after MSD transplants and UCBTs was scarce (0% and 9%, respectively). Notably, de novo autoimmunity is not a rare finding after HCT for primary immunodeficiencies occurring with MUD transplants.6
Myeloid DC >95% and >50% to 95% were associated with normal median platelet counts, whereas lower DC (5%-50%) yielded clearly lower (median, 40 × 109/L) platelet counts. These DC analyses are extremely useful for comparison because gene therapy trials are currently rarely achieving platelet numbers >100 × 109/L.5,7
Despite the general application of serotherapy, the incidence of grade 3 to 4 acute GVHD and chronic GVHD (15% and 17%, respectively) could have been lower, and there is no improvement over the results reported by Moratto et al3 (11.3% and 14.8%). Therapeutic drug monitoring of serotherapy, by serial measurements of plasma concentrations of antithymocyte globulin or alemtuzumab,8 may be one way to further reduce GVHD.
What would have happened if my anecdotal patient was diagnosed today and had chronic CMV infection? Volunteer donors in the registries have increased to ∼34 million, and the chance to find a suitable MUD has clearly improved. In the report of Burroughs et al, 63 patients received MUD grafts (9/10 and 10/10-HLA identical) and achieved satisfactory results. In case of unavailable MUDs/MSDs, haploidentical HCT by a CMV-seropositive relative using in vitro αβ T-cell receptor/CD19 depletion or in vivo postcyclophosphamide T-cell depletion is a reasonable alternative (see figure).9,10 However, larger studies are needed to prove their efficacy in competition with gene therapy.5
Conflict-of-interest disclosure: T.G. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal