Key Points
In SCD mice, inhibition of TF-initiated coagulation activation reduces microvascular stasis and neutrophil-platelet aggregates in lung.
Endothelial PAR-1 signaling contributes to the heme-induced microvascular stasis in a mouse model of SCD.
Abstract
Vaso-occlusive crisis (VOC) is the primary cause of morbidity and hospitalization in sickle cell disease (SCD); however, only 4 therapies (hydroxyurea, l-glutamine, crizanlizumab, and voxeletor) are currently approved in SCD. These agents limit the duration, severity, and frequency of crises. Activation of coagulation is a hallmark of SCD. Studies in animal models of SCD have shown that coagulation contributes to the chronic inflammation and end-organ damage associated with the disease; however, it is unknown whether coagulation directly contributes to the microvascular stasis that causes VOC. Herein, we demonstrate that inhibition of tissue factor (TF) and the downstream coagulation proteases factor Xa and thrombin significantly attenuates heme-induced microvascular stasis in mouse models of VOC. Pharmacologic inhibition of the principal thrombin receptor, protease activated receptor-1 (PAR-1), as well as deficiency of PAR-1 in all nonhematopoietic cells, also reduces stasis in sickle mice. PAR-1 deficiency was associated with reduced endothelial von Willebrand factor expression, which has been shown to mediate microvascular stasis. In addition, TF inhibition reduces lung vaso-occlusion in sickle mice mediated by arteriolar neutrophil-platelet microemboli. In sum, these results suggest that prophylactic anticoagulation might attenuate the incidence of VOC.
Introduction
Sickle cell disease (SCD) results in an altered erythrocyte physiology that causes vascular complications, such as hemolytic anemia, vaso-occlusion, chronic inflammation, and activation of coagulation.1 A hypercoagulable state and an increased risk for venous thromboembolism have been well documented in sickle patients.1-3 Several recent studies have identified coagulation activation as a mediator of disease pathology.4-6 Furthermore, it has been shown that activation of endothelial protease activated receptor-1 (PAR-1) with thrombin or agonist peptide enhances the interaction between sickle erythrocytes and endothelial cells, dependent on exocytosis of P-selectin (pSel) and von Willebrand factor (VWF) from endothelial Weibel-Palade bodies.7,8 Therefore, we hypothesized that tissue factor (TF)-mediated activation of coagulation might promote vaso-occlusion in SCD via thrombin-dependent PAR-1 activation on endothelial cells. We tested this hypothesis in 2 mouse models of vaso-occlusion: heme-induced stasis in skin microvasculature9 and lipopolysaccharide (LPS)-induced pulmonary vaso-occlusion mediated by arteriolar neutrophil-platelet microemboli.10
Study design
All animal experiments were approved by the University of North Carolina, University of Pittsburgh, and University of Minnesota Institutional Animal Care and Use Committees. We used male and female NY1DD11 and HbSS-Townes12 transgenic sickle mice. Generation of PAR-1+/+ and PAR-1−/− mice has been described previously.13 Detailed experimental methods are included in supplemental Materials and methods (available on the Blood Web site).
Results and discussion
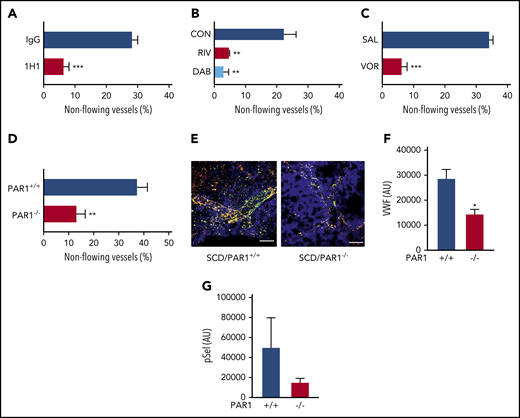
To determine whether coagulation contributes to microvascular stasis in sickle mice, we used a dorsal skinfold chamber model of vaso-occlusive crisis (VOC).9 In NY1DD mice pretreated with control immunoglobulin G, hemoglobin induced stasis in 28 ± 2.9% (mean ± standard deviation) of preselected vessels 1 hour after infusion. This was reduced significantly in NY1DD mice pretreated with an inhibitory anti-TF antibody 1H1 (Figure 1A). To determine whether TF contributes to stasis via generation of the downstream protease factor Xa or thrombin, NY1DD mice were treated with rivaroxaban or dabigatran, respectively. Similar to TF inhibition, factor Xa or thrombin inhibition also significantly reduced microvascular stasis 1 hour after hemoglobin infusion (Figure 1B). Previously, we demonstrated that the same doses of rivaroxaban and dabigatran efficiently anticoagulated sickle mice and reduced plasma levels of thrombin antithrombin complexes to the baseline levels observed in nonsickle mice.5 No spontaneous bleeding was observed; however, in a femoral vein bleeding model, hemostatic responses were significantly delayed by both anticoagulants.5 It is difficult to speculate what doses of these oral anticoagulants should be used to investigate their effect on the incidence of VOC in sickle cell patients. Importantly, 2 recent studies indicated that the approved doses of rivaroxaban were safely tolerated in sickle patients treated for venous thromboembolism, with noninferiority being demonstrated compared with warfarin.14,15
Inhibition of TF-dependent proteases and PAR-1 prevents heme-induced stasis in sickle mice. In sickle mice implanted with dorsal skinfold chambers, flowing venules were selected and mapped at baseline (20-25 venules per mouse). Mice were given a bolus infusion of stroma-free hemoglobin (SFH; 1.6 µmol/kg, IV). Percentage stasis was measured using intravital microscopy 1 hour after infusion. (A) NY1DD sickle mice were treated with control immunoglobulin G or anti-TF antibody 1H1 (25 mg/kg, intraperitoneally) 30 minutes prior to induction of stasis. (B) NY1DD mice received control chow, dabigatran (10 mg/g chow), or rivaroxaban (0.4 mg/g chow) for 4 days prior to induction of stasis. (C) NY1DD mice received saline or vorapaxar (150 µg/kg, oral gavage) once a day for 3 days prior to stasis experiments. (D) SCD/PAR1+/+ or SCD/PAR1−/− mice were infused with SFH 4 months after bone marrow transplantation. (E) The lungs of SCD/PAR1+/+ and SCD/PAR1−/− mice were excised after SFH infusion, fixed in 4% paraformaldehyde, and stained for surface pSel (green), VWF (red), and CD31 (blue). Costaining of green and red is shown as yellow. Quantification of positive pixels of VWF (scale bars, 30 μm) (F) and pSel (G) staining is represented as arbitrary units and was performed as described.9 Data are represented as mean percentage stasis + standard deviation, n = 3 to 5 mice per group. *P < .05, **P < .01, ***P < .001, 2-tailed, unpaired Student t test or 1-way analysis of variance, as appropriate.
Inhibition of TF-dependent proteases and PAR-1 prevents heme-induced stasis in sickle mice. In sickle mice implanted with dorsal skinfold chambers, flowing venules were selected and mapped at baseline (20-25 venules per mouse). Mice were given a bolus infusion of stroma-free hemoglobin (SFH; 1.6 µmol/kg, IV). Percentage stasis was measured using intravital microscopy 1 hour after infusion. (A) NY1DD sickle mice were treated with control immunoglobulin G or anti-TF antibody 1H1 (25 mg/kg, intraperitoneally) 30 minutes prior to induction of stasis. (B) NY1DD mice received control chow, dabigatran (10 mg/g chow), or rivaroxaban (0.4 mg/g chow) for 4 days prior to induction of stasis. (C) NY1DD mice received saline or vorapaxar (150 µg/kg, oral gavage) once a day for 3 days prior to stasis experiments. (D) SCD/PAR1+/+ or SCD/PAR1−/− mice were infused with SFH 4 months after bone marrow transplantation. (E) The lungs of SCD/PAR1+/+ and SCD/PAR1−/− mice were excised after SFH infusion, fixed in 4% paraformaldehyde, and stained for surface pSel (green), VWF (red), and CD31 (blue). Costaining of green and red is shown as yellow. Quantification of positive pixels of VWF (scale bars, 30 μm) (F) and pSel (G) staining is represented as arbitrary units and was performed as described.9 Data are represented as mean percentage stasis + standard deviation, n = 3 to 5 mice per group. *P < .05, **P < .01, ***P < .001, 2-tailed, unpaired Student t test or 1-way analysis of variance, as appropriate.
To determine whether thrombin contributes to stasis through activation of PAR-1, we used the PAR-1 inhibitor vorapaxar.16 Vorapaxar was developed as an inhibitor of human PAR-1; however, it also inhibits mouse PAR-1.17,18 In NY1DD mice treated with vehicle, microvascular stasis was observed in 34 ± 1.7% of preselected vessels. Importantly, vorapaxar treatment significantly attenuated microvascular stasis 1 hour after hemoglobin infusion (Figure 1C). In contrast to human platelets, mouse platelets do not express PAR-1, and thrombin-mediated platelet activation occurs via cleavage of PAR-3, with subsequent transactivation of PAR-4.19 Therefore, the protective effect of PAR-1 inhibition in sickle mice is independent of platelets and likely mediated by attenuation of PAR-1 signaling in endothelial cells. To confirm the role of endothelial PAR-1 in hemoglobin-induced stasis, we generated sickle mice that lack PAR-1 expression on all nonhematopoietic cells using bone marrow transplantation.5 Efficient bone marrow reconstitution was confirmed by hemoglobin electrophoresis (data not shown). Compared with sickle mice with normal expression of PAR-1, stasis was significantly reduced in sickle mice lacking PAR-1 on all nonhematopoietic cells (Figure 1D). Indeed, we found that this protection was associated with significantly reduced intensity of VWF staining, as well as a nonsignificant trend toward reduced pSel staining, in the lungs of sickle mice lacking PAR-1 (Figure 1E-G). We showed previously that inhibition of pSel or VWF markedly inhibits stasis in sickle mice.9 Together, these experiments indicate that thrombin contributes to stasis, in part, via activation of endothelial cell PAR-1. Interestingly, these results differ from our previous observation demonstrating that thrombin-dependent vascular inflammation in sickle cell mice was not attenuated by PAR-1 deficiency on all nonhematopoietic cells.5
Vorapaxar is approved by the US Food and Drug Administration as an antiplatelet agent for the treatment of myocardial infarction and peripheral arterial disease; however, it is contraindicated in patients with a history of stroke because of increased bleeding risk.16 Thus, it is unlikely to be used in sickle cell patients, who are at high risk to develop stroke. Importantly, however, this proof-of-concept study demonstrates that targeting PAR-1 has the potential to attenuate VOC in sickle patients. Future studies should explore alternative approaches to interrupt thrombin–PAR-1 signaling on endothelial cells (eg, by inducing biased PAR-1 signaling).20
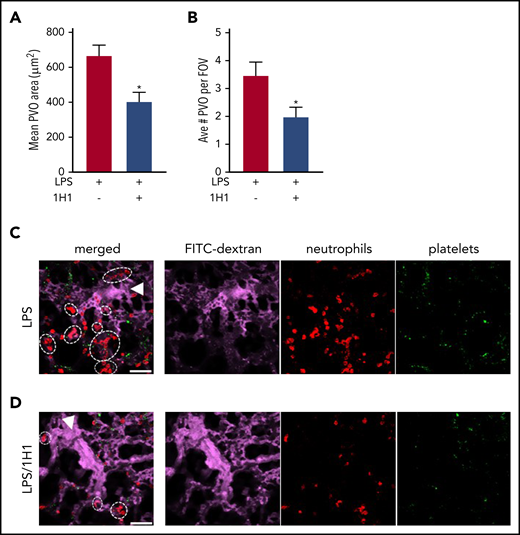
A recent finding suggests that gut microbiota–derived Toll-like receptor ligands can promote vaso-occlusion in SCD.21 Using quantitative fluorescent intravital lung microscopy (qFILM), we previously demonstrated pulmonary vaso-occlusion in SCD mice following IV challenge with a low-nanogram dose of LPS.10 Interestingly, unlike systemic vaso-occlusion, which never occurs in arterioles,22 pulmonary vaso-occlusion in sickle mice involved microembolism of precapillary pulmonary arterioles by large neutrophil-platelet aggregates.10 Importantly, inhibition of TF with 1H1 antibody attenuated pulmonary arteriole microembolism in sickle mice, as demonstrated by a reduced number of neutrophil-platelet aggregates and total aggregate area (Figure 2; supplemental Videos 1-4). These results highlight the therapeutic potential of targeting TF-initiated activation of coagulation to prevent systemic vaso-occlusion, as well as lung microembolism that might contribute to acute chest syndrome.10
TF inhibition attenuates formation of LPS-induced pulmonary vaso-occlusions in SCD mice. The effect of anti-TF 1H1 antibody (5 mg/kg, IV) on the formation of neutrophil/platelet microemboli. 1H1 was administered 30 minutes prior to infusion with LPS (0.1 µg/kg, IV). Arterioles were imaged 2 to 2.5 hours after LPS infusion using qFILM. The mean pulmonary arteriolar vaso-occlusion (PVO) area (A) and average number of PVO per field of view (FOV) (B) were quantified in control sickle mice (black) or sickle mice treated with 1H1 (gray). n = 5 mice per group. Representative qFILM images of lungs from a control sickle mouse (C) or from a sickle mouse treated with 1H1 (D). The far left panels represent merged images of the circulation (FITC-dextran, purple), neutrophils (red), and platelets (green). Arrowheads denote the direction of blood flow, and dashed ovals and circles denote PVOs. Scale bars, 50 µm. *P < .05, 2-tailed, unpaired Student t test.
TF inhibition attenuates formation of LPS-induced pulmonary vaso-occlusions in SCD mice. The effect of anti-TF 1H1 antibody (5 mg/kg, IV) on the formation of neutrophil/platelet microemboli. 1H1 was administered 30 minutes prior to infusion with LPS (0.1 µg/kg, IV). Arterioles were imaged 2 to 2.5 hours after LPS infusion using qFILM. The mean pulmonary arteriolar vaso-occlusion (PVO) area (A) and average number of PVO per field of view (FOV) (B) were quantified in control sickle mice (black) or sickle mice treated with 1H1 (gray). n = 5 mice per group. Representative qFILM images of lungs from a control sickle mouse (C) or from a sickle mouse treated with 1H1 (D). The far left panels represent merged images of the circulation (FITC-dextran, purple), neutrophils (red), and platelets (green). Arrowheads denote the direction of blood flow, and dashed ovals and circles denote PVOs. Scale bars, 50 µm. *P < .05, 2-tailed, unpaired Student t test.
Despite the well-described hypercoagulable state, only 1 adequately powered placebo-controlled study has investigated the effect of anticoagulation in SCD, using the low molecular weight heparin tinzaparin. Once-daily administration of tinzaparin (175 IU/kg) upon admission for VOC significantly reduced the duration of painful crisis and hospital stay.23 It has been demonstrated that the concentrations of low molecular weight heparins used clinically to achieve anticoagulation are not sufficient to effectively block pSel.24 Therefore, it is likely that protection provided by tinzaparin was mediated by its anticoagulant properties rather than the direct inhibition of pSel‐mediated cellular interactions.25,26 This notion is further supported by 2 recent clinical trials. The first investigated the effects of sevuparin, a heparin derivative that retains antiadhesive (anti-pSel) properties but has very low anticoagulant activity (NCT02515838).27 In preclinical models, sevuparin reduced sheep red blood cell adhesion to endothelium and reduced microvascular stasis in animal models27 ; however, daily administration to sickle patients upon admission for painful crisis did not improve resolution of VOC.28 Similar negative results were reported for rivipansel, a pan-selectin inhibitor.29 This is in contrast to the pSel-neutralizing antibody crizanlizumab, which reduced the incidence of VOC in sickle patients when given prophylactically.30 Together with our results, the outcomes of these clinical trials suggest that anticoagulants may reduce the incidence and, possibly, duration of VOC,23 whereas targeting pSel is only effective in preventing VOCs.
As with all preclinical studies using animal models, one should be careful about overinterpreting results until appropriate clinical trials confirm basic research observations. The single time point used to assess VOC and treatments prior to VOC induction are the main limitations of our study; however, the robust protection reported in our study, together with the limited range of therapeutic options for SCD patients in VOC, highlights the need to investigate anticoagulants as a possible treatment strategy.
For original data, please contact Rafal Pawlinski (rafal_pawlinski@med.unc.edu) or John D. Belcher (belcher@umn.edu).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01 HL142604, R01 HL114567, R01 HL128297, R01 HL141080, and T32 HL007149.
Authorship
Contribution: E.M.S. designed research, performed research, analyzed data, and wrote the manuscript; C.C., T.B., J.N., and S.W. performed research; P.S. and G.M.V. provided analytical tools; N.S.K. designed research and edited the manuscript; J.D.B. designed research and provided analytical tools; and R.P. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rafal Pawlinski, UNC Blood Research Center, Division of Hematology/Oncology, Department of Medicine, University of North Carolina at Chapel Hill, 116 Manning Dr, 8008C Mary Ellen Jones Building, Chapel Hill, NC 27599; e-mail: rafal_pawlinski@med.unc.edu; and John D. Belcher, Division of Hematology, Oncology and Transplantation, Department of Medicine, University of Minnesota, 420 Delaware St SE, MMC 480, Minneapolis, MN 55455; e-mail: belcher@umn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal