In this issue of Blood, Arenas et al1 report that a group of patients suffering from idiopathic multicentric Castleman disease (iMCD) display heightened activation of the mTOR signaling pathway, suggesting a much-needed new treatment strategy for this disease. iMCD is a rare but deadly hematologic disorder characterized by a slew of abnormalities, including cytokine-driven lymphoproliferation and lymph node enlargement, systemic inflammation, cytopenias, and multiorgan dysfunction.2,3 The most severe cases of iMCD, known collectively as the TAFRO subtype, also display thrombocytopenia, widespread edema, fever, and organomegaly.4 The causes of iMCD and what propagates the disease remain largely unknown, leaving patients with few treatment options. An important advance was the identification of the proinflammatory cytokine interleukin-6 (IL-6) as a driver of iMCD.5 This observation led to the approval and use of biologics that block IL-6 signaling as a treatment of iMCD. Although targeting IL-6 has met with some success, ∼60% of iMCD patients treated with antagonizers of IL-6 signaling failed to respond for reasons that remain unknown.6 These findings suggest mechanisms in addition to or beyond IL-6 signaling drive the etiology and pathogenesis of iMCD. Identification of such mechanisms is a critical need, as it could lead to new and more effective treatments for iMCD.
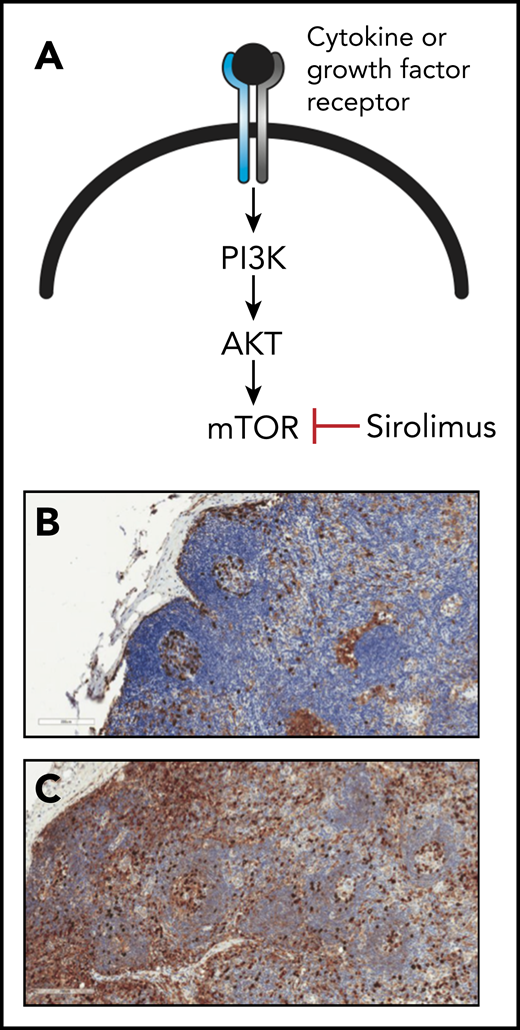
The activity of the mTOR signaling pathway is elevated in lymph nodes from patients with iMCD. (A) Schematic diagram outlining the mTOR signaling pathway, which is activated by engagement of cytokine or growth factor receptors with their respective ligands. The mTOR inhibitor sirolimus is used clinically to block mTOR signaling. (B-C) Lymph node sections from a control patient (B) or a patient with iMCD (C). Sections were stained with antibodies specific for the phosphorylated form of the ribosomal protein S6 (pS6; brown color), which becomes phosphorylated in response to mTOR signaling. Note the elevated levels of pS6 in the lymph node section from the iMCD patient relative to that from control, which signifies greater activation of mTOR signaling. The blue color represents staining of cell nuclei with hematoxylin. Original magnification ×20 for panels B-C. See Figure 1 in the article by Arenas et al that begins on page 1673.
The activity of the mTOR signaling pathway is elevated in lymph nodes from patients with iMCD. (A) Schematic diagram outlining the mTOR signaling pathway, which is activated by engagement of cytokine or growth factor receptors with their respective ligands. The mTOR inhibitor sirolimus is used clinically to block mTOR signaling. (B-C) Lymph node sections from a control patient (B) or a patient with iMCD (C). Sections were stained with antibodies specific for the phosphorylated form of the ribosomal protein S6 (pS6; brown color), which becomes phosphorylated in response to mTOR signaling. Note the elevated levels of pS6 in the lymph node section from the iMCD patient relative to that from control, which signifies greater activation of mTOR signaling. The blue color represents staining of cell nuclei with hematoxylin. Original magnification ×20 for panels B-C. See Figure 1 in the article by Arenas et al that begins on page 1673.
The work by Arenas et al substantially extends a prior but limited study performed by the same group that characterized blood and tissue samples from 3 iMCD-TAFRO patients who failed to respond to blockade of IL-6 signaling.7 This analysis identified elevated mTOR signaling as a hallmark of iMCD-TAFRO. The mTOR signaling pathway is activated by numerous growth factors and cytokines and controls several facets of cell physiology, including protein synthesis, proliferation, metabolism, and survival.8 The association of increased mTOR signaling with iMCD-TAFRO prompted treatment of the 3 patients with sirolimus, an mTOR inhibitor used clinically to suppress organ transplant rejection. Strikingly, this approach led to durable disease remission in all 3 patients. A critical unanswered question arising from these studies was whether heightened mTOR signaling is a general characteristic of all iMCD-TAFRO patients or only a subset.
To address this question, Arenas et al assessed the activity of the mTOR signaling pathway in a larger cohort of iMCD-TAFRO patients. Consistent with findings from the smaller study, Arenas et al report that several indicators of mTOR signaling were significantly elevated in samples from the larger group of iMCD-TAFRO patients compared with those from controls (see figure). Importantly, mTOR pathway activation in iMCD-TAFRO patient samples was significantly higher than that observed in samples from patients suffering from other diseases associated with lymphoproliferation, but similar to that seen in patients with ALPS (autoimmune lymphoproliferative syndrome), a disease known to be driven by mTOR signaling that is treatable with mTOR inhibitors. Hyperactive mTOR signaling was apparent in several immune cell types, which is consistent with the idea that iMCD is driven by immune system dysregulation. Surprisingly, however, T cells accounted for only a small proportion of cells with increased mTOR signaling, an unexpected finding given that this cell type likely has a central role in the etiology and pathogenesis of iMCD. Increased mTOR signaling was also tied to hyperresponsiveness to IL-6 in immune cells from iMCD-TAFRO patients, suggesting that elevation of mTOR signaling is, at least in part, due to the action of the IL-6 signaling pathway. These data prompt a key question: why is blocking IL-6 an ineffective treatment for such a large proportion of iMCD patients? One explanation could be that components of the IL-6 signaling pathway (or other cytokine signaling pathways) that act inside of cells are disrupted or altered by mutation, making therapies that target exterior components of the IL-6 signaling pathway (ie, IL-6 itself and its receptor) less effective. Cell-intrinsic molecules that might be altered and lead to hyperactive cytokine signaling could be the JAK kinases, which promote signaling by cytokines, or SOCS1 or 3, which are proteins that act in a negative feedback loop to suppress cytokine signaling. This prompts the larger issue of identifying additional cytokines, growth factors, and cellular pathways besides IL-6 signaling that contribute to iMCD, which could provide valuable information regarding not only the etiology of iMCD but also additional potential therapeutic targets. One approach to this may to be carry out genome-wide association studies by exome- or whole-genome sequencing of samples from iMCD patients.
The studies by Arenas et al are a prime example of how discovery by personalized medicine can lead to critical breakthroughs in understanding diseases that lack animal models or other investigative tools. Moreover, in this case, the analysis of patient samples has uncovered the possibility of using sirolimus or other mTOR inhibitors as a treatment of iMCD, which is currently being tested in a clinical trial. A key unknown is whether iMCD patients will show a better response rate to mTOR inhibition than to blockade of IL-6 signaling. The hope would be that the vast majority of iMCD patients respond well to mTOR inhibition. However, if only a subset of patients respond to blockade of mTOR signaling, then it will be crucial to identify specific cytokines and growth factors besides IL-6 that may contribute to iMCD as suggested above. Also, what cell types manifesting heightened mTOR signaling are responsible for driving disease pathogenesis remains unknown; defining these could be important for diagnostic purposes as well as for identifying more specific therapies for iMCD.
Conflict-of-interest disclosure: J.D.C. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal