TO THE EDITOR:
Continuous treatment with tyrosine kinase inhibitors (TKIs) induces a fast initial response and leads to long-term remission in most patients with chronic myeloid leukemia (CML).1 Consequently, the annual mortality in CML has decreased to 1% to 2%,2 and patients with CML approach the life expectancy of the general population.3 However, continuous treatment with TKI is costly and associated with significant side effects. Therefore, alternative treatment strategies are needed. Although TKI dose reductions during established molecular remission have only been considered recently,4,5 the potential of TKI treatment cessation for patients with CML in sustained molecular remission has been studied in greater detail.6,7 Several studies consistently report that ∼50% of patients with CML remain in treatment-free remission (TFR) for many years, whereas most of the other patients present with recurring disease levels after stopping TKI.8,9 Although TKI treatment duration and time in molecular remission are associated with higher TFR rates,9 a reliable prospective prediction of disease recurrence for individual patients is not possible yet. The DESTINY trial (#NCT01804985) differs from other TKI stop studies in that TKI treatment is reduced to 50% of the standard dose for 12 months prior to cessation, including 125 patients in stable MR4 (BCR-ABL1IS < 0.01%) and 49 patients in stable MR3 (BCR-ABL1IS < 0.1%) but not MR4 before entry. The trial demonstrates that this strategy improves the fraction of MR4 patients in successful TFR to >70% at 2 years after treatment cessation, whereas also ∼36% of MR3 patients retained TFR.10,11 Here, we evaluate the BCR-ABL1IS values monitored during dose reduction and suggest that they can serve as a predictor of individual CML recurrence after TKI cessation independent of the BCR-ABL1IS values at study entry.
BCR-ABL1IS levels are regularly obtained from peripheral blood cells of patients with CML to monitor their leukemia load. Within DESTINY, BCR-ABL1IS was available for all 174 patients measured prior to dose reduction (time point 0), monthly during the 12-month dose reduction period, and monthly or every second month thereafter until month 36. Molecular recurrence was defined as the first of 2 consecutive BCR-ABL1IS measurements >0.1% or using particular case reports indicating recurrences without achieving BCR-ABL1IS > 0.1%. In total, 67 recurrences were observed of which 12 occurred during the dose reduction period. At recurrence, TKI treatment at full dose was reinitiated. In order to quantify the change of BCR-ABL1IS during the 12-month dose reduction period, we applied a linear regression to each patient and estimated the individual slope measured on the log[BCR-ABL1IS] scale (Figure 1A), if at least 3 nonzero BCR-ABL1IS values were available. Thereby, we excluded 3 of 174 patients. The correlation of individual slopes and molecular recurrence was quantified by logistic regression analysis.
BCR-ABL1IS level monitored during dose reduction is predictive for individual CML recurrence after TKI cessation. (A) BCR-ABL1IS dynamics of 2 patients upon dose reduction. The left example corresponds to a patient with a low positive slope of the BCR-ABL1IS values during the dose reduction period (green line indicates the slope) that remains in TFR after therapy stop. The right example illustrates a patient who is characterized by a high slope during the 12-month dose reduction period (red line) and presents with a recurrence after TKI stop (red dots). (B) Histogram for patient-specific estimates of the individual BCR-ABL1IS slope during the 12-month dose reduction period (n = 171). The 95%-quantile (red dashed line) of the normal distribution fitted to patients with negative/low slope (green curve) is taken as a cutoff parameter (at 0.068 log[BCR-ABL1IS] per month) to separate patients with high slopes (red curve). (C) Logistic regression curve for patient-specific estimates of the individual BCR-ABL1IS slope during the 12-month dose reduction period (n = 171) with corresponding OR and 95% CI per 0.01 log-increase per month. Patients are separated into cohorts with negative/low slopes (green area) and patients with high slopes (red area) by applying the cutoff parameter announced in panel B. (D) Fraction of patients with and without recurrence in the cohorts with negative/low and high slopes during dose reduction (n = 171) and corresponding OR with 95% CI.
BCR-ABL1IS level monitored during dose reduction is predictive for individual CML recurrence after TKI cessation. (A) BCR-ABL1IS dynamics of 2 patients upon dose reduction. The left example corresponds to a patient with a low positive slope of the BCR-ABL1IS values during the dose reduction period (green line indicates the slope) that remains in TFR after therapy stop. The right example illustrates a patient who is characterized by a high slope during the 12-month dose reduction period (red line) and presents with a recurrence after TKI stop (red dots). (B) Histogram for patient-specific estimates of the individual BCR-ABL1IS slope during the 12-month dose reduction period (n = 171). The 95%-quantile (red dashed line) of the normal distribution fitted to patients with negative/low slope (green curve) is taken as a cutoff parameter (at 0.068 log[BCR-ABL1IS] per month) to separate patients with high slopes (red curve). (C) Logistic regression curve for patient-specific estimates of the individual BCR-ABL1IS slope during the 12-month dose reduction period (n = 171) with corresponding OR and 95% CI per 0.01 log-increase per month. Patients are separated into cohorts with negative/low slopes (green area) and patients with high slopes (red area) by applying the cutoff parameter announced in panel B. (D) Fraction of patients with and without recurrence in the cohorts with negative/low and high slopes during dose reduction (n = 171) and corresponding OR with 95% CI.
We observed that the majority of patients had either negative slopes (ie, BCR-ABL1IS decreasing) or low positive slopes close to zero (Figure 1B), indicating that their BCR-ABL1IS levels did not rise substantially, if at all, during the dose reduction period. In contrast, there is a group of patients with considerably higher slopes, indicating an increase in BCR-ABL1IS. Logistic regression analysis shows that for an additional 0.01 log[BCR-ABL1IS] increase per month in the slope parameter, there was a 28% increased chance of recurrence (odds ratio [OR]: 1.28; 95% confidence interval [CI]: 1.17-1.42) at any time (Figure 1C). This also applies if the MR3 and MR4 subgroups are analyzed separately (supplemental Figure 1, available on the Blood Web site) and indicates that an increase of BCR-ABL1IS during the dose reduction period is strongly associated with eventual molecular recurrence.
We identified the 95%-quantile of a normal distribution fitted to the negative/low BCR-ABL1IS slopes at 0.068 log[BCR-ABL1IS] increase per month as a suitable cutoff to split the patient cohort into 80.7% with negative/low slopes and 19.3% with high slopes (Figure 1B; supplemental material). Irrespective of the response level prior to dose reduction, 72.5% of the patients presenting with a negative/low slope during dose reduction remained recurrence free at 2 years after stopping treatment. In the high slope group, 87.9% of the patients had disease recurrence, whereas only 4 patients (12.1%) did not report the adverse event, of which 3 were censored. The OR calculated for the categorized patients is 19.1 (95% CI: 6.3-57.9; Figure 1D), indicating that the risk of recurrence is dramatically increased for the high slope group compared with the group with negative/low slopes.
Almost all TFR studies hitherto have focused on patients in stable MR4, whereas the DESTINY trial also included 49 MR3 patients. Having a closer look at their response during dose reduction separately, it appears that 30.6% of the MR3 patients presented with high slopes, whereas only 14.8% of the patients in MR4 showed similarly elevated slopes. For instance, patients in MR3 have a 2.6-fold higher chance to present with highly increasing BCR-ABL1IS levels during dose reduction compared with patients in MR4 (OR: 2.6; 95% CI: 1.2-5.6).
Although less common than with high slopes, recurrences after stopping TKI nevertheless occurred in patients with negative/low slopes during the dose reduction period in both the MR3 and the MR4 cohorts. A detailed analysis of the distribution of the individual slopes of those patients (Figure 2A) reveals that there is a tendency for lower slopes in the remission cohort as compared with the recurrence cohort, thereby underpinning our findings of the overall logistic regression model.
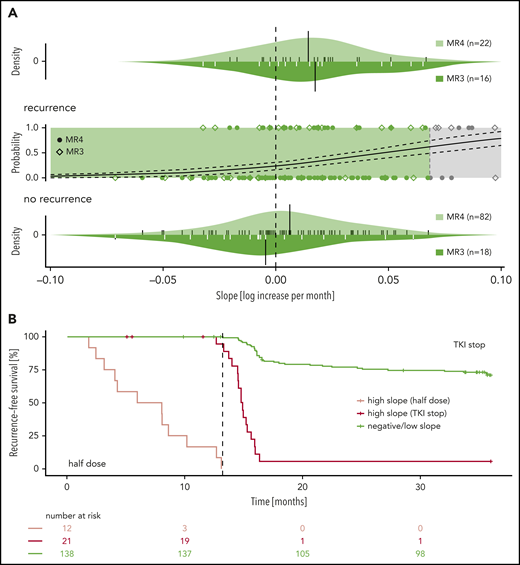
Subgroup and recurrence-free survival analysis. (A) Subgroup analysis of the patients with negative/low slope (n = 138): The central part reproduces an inset of the logistic regression curve for patient-specific estimates of the individual BCR-ABL1IS slope during the 12-month dose reduction period. Patients are categorized according to their prior BCR-ABL1IS level as either MR3 (rhombi) or MR4 (filled circles). The upper and lower panels depict the slope distributions of patients with and without molecular recurrence, respectively, stratified for the molecular response level before dose reduction (ie, MR3: dark shaded; MR4: light shaded). Small ticks indicate the estimates of the individual BCR-ABL1IS slopes, and black bars illustrate the mean of the slope of the respective group (upper panel: mean of MR3 recurrence group = 0.017 and MR4 recurrence group = 0.014; lower panel: mean of MR3 nonrecurrence group = −0.005 and MR4 nonrecurrence group = 0.006). (B) Recurrence-free survival of patients with either negative/low (green) or high slope (red) during the 12-month reduction period (n = 171). The patients with high slopes are further separated as to whether they present with disease recurrence during the half dose period (light red) or after treatment stop.
Subgroup and recurrence-free survival analysis. (A) Subgroup analysis of the patients with negative/low slope (n = 138): The central part reproduces an inset of the logistic regression curve for patient-specific estimates of the individual BCR-ABL1IS slope during the 12-month dose reduction period. Patients are categorized according to their prior BCR-ABL1IS level as either MR3 (rhombi) or MR4 (filled circles). The upper and lower panels depict the slope distributions of patients with and without molecular recurrence, respectively, stratified for the molecular response level before dose reduction (ie, MR3: dark shaded; MR4: light shaded). Small ticks indicate the estimates of the individual BCR-ABL1IS slopes, and black bars illustrate the mean of the slope of the respective group (upper panel: mean of MR3 recurrence group = 0.017 and MR4 recurrence group = 0.014; lower panel: mean of MR3 nonrecurrence group = −0.005 and MR4 nonrecurrence group = 0.006). (B) Recurrence-free survival of patients with either negative/low (green) or high slope (red) during the 12-month reduction period (n = 171). The patients with high slopes are further separated as to whether they present with disease recurrence during the half dose period (light red) or after treatment stop.
Our findings suggest an amended strategy for TKI cessation studies, exploiting additional information from intermediate dose reduction: After the 12-month dose reduction period, patients should only be stopped if they are still below MR3 (ie, no early recurrence) and additionally show a negative/low BCR-ABL1IS slope during this period. Patients with high slopes should return to full-dose TKI treatment, as a future recurrence is extremely likely and they do not even sufficiently manage the half-dose reduction. Enforcing such a strategy within the DESTINY protocol could have further increased the fraction of recurrence-free patients among those patients who stopped TKI after the 12-month dose reduction (Figure 2B). For completeness, we note that patients with continuing negative BCR-ABL1IS measurements are rare within DESTINY but might occur more often in other cessation studies with more stringent entry criteria. Although we exclude such patients (n = 3) from our analysis, this cohort might have to be considered separately in future studies. However, as long as no measurable increase of BCR-ABL1IS levels can be detected, those patients are not considered as high risk anyway.
From our analysis, we conclude that the individual molecular dynamics during TKI dose reduction is a promising predictor for a molecular recurrence after TKI cessation. In particular, the exclusion of patients with highly increasing BCR-ABL1IS slopes during the dose reduction period from complete TKI cessation is likely to reduce the overall number of recurrences and to increase the TFR success rate on stopping TKI. For the clinical decision about whether to stop TKI treatment after the dose reduction period, we have estimated the positive predictive value (ie, probability of losing TFR for patients with high slope) and the negative predictive value (ie, probability of keeping TFR for patients with negative/low slope) to be 81.0% and 72.5%, respectively, based on the 159 DESTINY patients at risk at month 12 and a recurrence prevalence of 34.6%. Conceptually, our results demonstrate that frequent monitoring of molecular response during dose reduction can inform patient management by minimizing the risk for CML recurrence on a subsequent TFR attempt. A validation of our quantitative estimates using an independent cohort is necessary to confirm our predictions and to compare or complement our approach with further biomarkers of TFR.
Data of the DESTINY trial are already presented in Clark et al.10,11
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by the German Federal Ministry of Education and Research (www.bmbf.de/en/), grants 031A424 “HaematoOpt” (I.R.), 031A315 “MessAge” (I.G.), ERA-Net ERACoSysMed JTC-2 project “prediCt” 031L0136A (I.R.), and Bloodwise grant 13020 (R.E.C.).
Authorship
Contribution: R.E.C. and S.C. provided the data; A.G., I.G., and I.R. analyzed the data; A.G., I.G., R.E.C., and I.R. wrote the paper.
Conflict-of-interest disclosure: I.G. received travel and research funding from Bristol-Myers Squibb (BMS). I.R. received honorarium, travel, and research funding from BMS; and honorarium from Janssen-Cilag. R.E.C. received research support and honoraria from Pfizer, Novartis, and BMS, and honoraria from Ariad/Incyte, Abbvie, and Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Ingo Roeder, Institute for Medical Informatics and Biometry, Faculty of Medicine Carl Gustav Carus, Technische Universität Dresden, Fetscherstr. 74, D-01307 Dresden, Germany; e-mail: ingo.roeder@tu-dresden.de.
REFERENCES
Author notes
A.G. and I.G. contributed equally.
R.E.C. and I.R. contributed equally.

![BCR-ABL1IS level monitored during dose reduction is predictive for individual CML recurrence after TKI cessation. (A) BCR-ABL1IS dynamics of 2 patients upon dose reduction. The left example corresponds to a patient with a low positive slope of the BCR-ABL1IS values during the dose reduction period (green line indicates the slope) that remains in TFR after therapy stop. The right example illustrates a patient who is characterized by a high slope during the 12-month dose reduction period (red line) and presents with a recurrence after TKI stop (red dots). (B) Histogram for patient-specific estimates of the individual BCR-ABL1IS slope during the 12-month dose reduction period (n = 171). The 95%-quantile (red dashed line) of the normal distribution fitted to patients with negative/low slope (green curve) is taken as a cutoff parameter (at 0.068 log[BCR-ABL1IS] per month) to separate patients with high slopes (red curve). (C) Logistic regression curve for patient-specific estimates of the individual BCR-ABL1IS slope during the 12-month dose reduction period (n = 171) with corresponding OR and 95% CI per 0.01 log-increase per month. Patients are separated into cohorts with negative/low slopes (green area) and patients with high slopes (red area) by applying the cutoff parameter announced in panel B. (D) Fraction of patients with and without recurrence in the cohorts with negative/low and high slopes during dose reduction (n = 171) and corresponding OR with 95% CI.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/135/10/10.1182_blood.2019003395/2/m_bloodbld2019003395f1.png?Expires=1769203089&Signature=Ti~pA2k5J15-9HotVF4diR9pYnnbMdzFZrH4PbhUXLjRPoLD81Jt52~MdLCDSHNeWSii98J1yMdc3DLfm4E-p53hK4ZnoKGfYmma4jGQdQSuITaFXnmYlZPbvuNptmcpd4Lkk8hmYeUldA789fImZEAfljYKfqiEABoY-AzxlVen2F253MnxlTNWH9~zQkRWLREcblrB8U3vG6mkm-96I4t3zwUT46F6Rc827ie5JTrElHeJYYvEC~W~2q2MZEBU3it1pGvjy5ZOHq4HDhjeqDlvpL3HFWpFOgCxuymgkQBJYwzJ95ZAPyMyihaux-qGOs-GvYZMVfIx5iK4fpJHJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal