
Background: AMG 673 is a novel half-life extended (HLE) BiTE® (bispecific T-cell engager) construct that binds both CD33 and CD3 and is genetically fused to the N-terminus of a single-chain IgG Fc region, thereby potentially increasing the half-life of the molecule. AMG 673 redirects T cells toward CD33+ cells, with the induced proximity leading to T-cell‒mediated cytotoxicity against acute myeloid leukemia (AML) blasts. Anti-AML activity of other CD33/CD3 bispecific T-cell engager molecules has been previously reported (Blood, 2018, 132, 25; Blood, 2018, 132, 1455). The objectives of this ongoing study are to evaluate the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD), and preliminary efficacy of AMG 673 in adult patients aged ≥18 years with relapsed/refractory (R/R) AML.
Methods: This is an ongoing first-in-human, open-label, phase 1, sequential dose escalation study (NCT03224819). AMG 673 was administered as two, short, and intermittent intravenous (IV) infusions during a 14-day cycle in adult patients with R/R AML. Patients received treatment cycles of AMG 673 until disease progression or unacceptable toxicities. T-cell activation, cytokine, and AMG 673 levels in patients' blood were evaluated by validated assays. Results were summarized descriptively by the dosing cohorts and potential associations between PK, PD, safety, and preliminary efficacy were evaluated.
Results: As of June 14, 2019, 30 patients had enrolled in 10 cohorts and were treated with AMG 673 (dose range, 0.05-72 μg IV per dose). The median age was 67.5 (range: 25.0-84.0) years; 20/30 (67%) patients had received ≥4 prior anti-AML treatments, baseline myelosuppression at study entry was common (grade ≥3 neutropenia 21/30 [70%], thrombocytopenia 25/30 [83%], leukopenia 14/30 [47%]), and 7/30 (23%) patients had undergone hematopoietic stem cell transplant (HSCT) before enrolling in the study.
Patients received a median of 1.5 (range: 1.0-6.0) cycles of AMG 673; 27/30 (90%) patients discontinued treatment due to disease progression (n=21), patient request (n=2), protocol-specified criteria (n=2), or adverse events (AEs; n=2). A total of 3 patients were still receiving AMG 673 at the time of data analysis. The most common treatment-related AE was cytokine release syndrome (CRS) reported in 15/30 (50%) patients (grade 1, n=6; grade 2, n=5; grade 3, n=4; no grade 4 CRS). Treatment-related serious AEs were reported in 11/30 (37%) patients, and 15/30 (50%) patients experienced treatment-related AEs of grade ≥3, with the most common being abnormal hepatic enzymes (n=5, 17%), CRS (n=4, 13%), leukopenia (n=4, 13%), thrombocytopenia (n=2, 7%), and febrile neutropenia (n=2, 7%). Two deaths, unrelated to AMG 673, were reported on days 19 and 28 after the last dose.
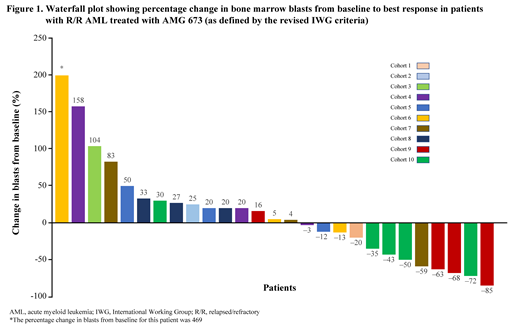
Assessment of bone marrow in treated patients showed a decrease in blasts in 12/27 (44%) evaluable patients, of which 6 experienced ≥50% reduction in blasts compared with baseline (Figure 1). One patient achieved complete remission with incomplete hematologic recovery (CRi) with 85% reduction in bone marrow blasts at a dose of 36 µg.
Dose-related increases in Cmax and AUC were observed following AMG 673 infusions. Preliminary half-life estimates for AMG 673 were longer than those observed for canonical CD33-specific BiTE® molecule with short half-lives. Upregulation of T-cell activation markers CD25 and CD69 on T-cell subsets and cytokine release post-infusion were observed at higher doses. Preliminary associations between AMG 673 exposures, T-cell activation, safety, and clinical response have been evaluated.
Conclusions: Preliminary data of AMG 673 dosed up to 72 µg provide early evidence of the molecule's acceptable safety profile, drug tolerability, and anti-leukemic activity. An association was observed between PK/PD relationships that were consistent with the biological activity of AMG 673. These preliminary results support further dose escalation of the AMG 673 HLE BiTE® molecule in patients with R/R AML.
Subklewe:AMGEN: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Morphosys: Research Funding; Janssen: Consultancy; Celgene: Consultancy, Honoraria; Miltenyi: Research Funding; Oxford Biotherapeutics: Research Funding; Pfizer: Consultancy, Honoraria. Stein:Celgene: Speakers Bureau; Stemline: Speakers Bureau; Amgen: Consultancy, Speakers Bureau. Walter:Amgen: Consultancy; Amphivena Therapeutics: Consultancy, Equity Ownership; Aptevo Therapeutics: Consultancy, Research Funding; Argenx BVBA: Consultancy; Astellas: Consultancy; BioLineRx: Consultancy; BiVictriX: Consultancy; Seattle Genetics: Research Funding; Boehringer Ingelheim: Consultancy; Boston Biomedical: Consultancy; Covagen: Consultancy; Daiichi Sankyo: Consultancy; Agios: Consultancy; Race Oncology: Consultancy; Jazz Pharmaceuticals: Consultancy; Kite Pharma: Consultancy; Pfizer: Consultancy, Research Funding; New Link Genetics: Consultancy. Wei:Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Macrogenics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Honoraria, Research Funding; Janssen: Honoraria; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: AHW is a former employee of the Walter and Eliza Hall Institute and receives a fraction of its royalty stream related to venetoclax, Research Funding, Speakers Bureau. Ritchie:Amgen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy; BMS: Research Funding; Takeda: Research Funding; Beigene: Research Funding; Imago: Research Funding; Novartis: Honoraria; Sanofi: Honoraria. Vachhani:AbbVie: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Agios: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Astellas: Speakers Bureau. Dai:Amgen: Employment, Equity Ownership. Hindoyan:Amgen Inc.: Employment, Other: stock ownership. Agarwal:Amgen: Employment, Equity Ownership; AbbVie: Equity Ownership. Anderson:Amgen Inc.: Employment, Equity Ownership. Khaldoyanidi:Amgen: Employment, Equity Ownership; BMS: Equity Ownership. Ravandi:Macrogenix: Consultancy, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Consultancy, Research Funding; Menarini Ricerche: Research Funding; Selvita: Research Funding; Cyclacel LTD: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal