Successful hematopoietic cell transplantation (HCT) requires vacating recipient hematopoietic stem cell (HSC) niches to permit transplanted HSC to engraft. Currently, DNA damaging radiation or chemotherapy are used to eliminate recipient HSC and achieve niche clearance. We have pursued a non-genotoxic approach to target and deplete HSC using a humanized monoclonal antibody, AMG 191, that binds human CD117 (c-Kit), a receptor tyrosine kinase expressed on the surface of HSC and progenitor cells (HSPC). We have shown that AMG 191 suppresses human hematopoiesis in vitro, depletes human HSC in mice xenografted with human cells, and safely depletes HSC of non-human primates. We have initiated a Phase I dose escalation trial to test AMG 191 as the sole conditioning agent to achieve donor CD34-enriched HSPC engraftment in patients undergoing HCT for severe combined immunodeficiency (SCID) (ClinicalTrials.gov: NCT02963064). SCID is a severe genetic immune disorder curable only by HCT. Because of toxicity concerns, infants with SCID often receive donor hematopoietic grafts without conditioning, resulting in a lack of donor HSC engraftment. Instead, mature T lymphocytes and possibly lymphoid progenitors engraft but support only donor T cell development. This approach is associated with incomplete and poorly sustained immune reconstitution, and many patients have either no donor B cells and/or poor B cell function requiring life-long immunoglobulin replacement therapy. Second unconditioned donor HSC "boosts" can be performed, but they do not result in HSC engraftment and immune defects may persist.
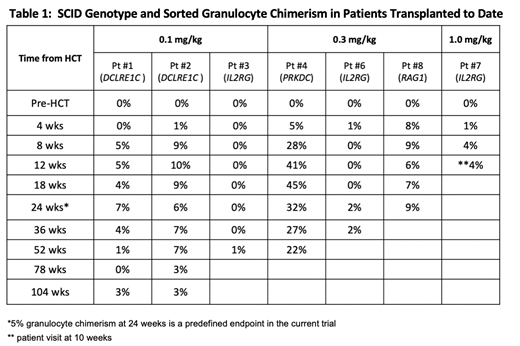
The primary endpoint of our study is to assess the safety and tolerability of AMG 191 as a conditioning agent in SCID patients. Secondary endpoints include AMG 191 pharmacokinetics (PK), host HSC depletion, and the determination of the dose of AMG 191 that achieves adequate donor HSC engraftment, defined as >5% donor blood granulocyte chimerism in peripheral blood at 24 weeks. Seven patients have been treated to date who are >12 weeks post-HCT (Table 1): three in each of the first two dose cohorts (0.1 and 0.3 mg/kg AMG 191), and one patient in the third cohort (1.0 mg/kg). An eighth patient has been treated at the 1.0 mg/kg dose and is three weeks post-HCT. Patients have a mixture of SCID genotypes. All patients treated to date had prior HCT with lack of donor HSC engraftment as evidenced by 0% donor sorted granulocyte chimerism at study entry. AMG 191 administration and infusion of original donor CD34+-selected cells were uniformly well tolerated. Pre- and post-infusion marrow analyses in five evaluable patients demonstrated dose-dependent decline in CD117+HSPC following AMG 191 treatment. Table 1 shows that four of six patients, who are >24 weeks post-HCT, reached the predefined endpoint of >5% granulocyte chimerism at 24 weeks, demonstrating donor HSC engraftment. The two patients who did not show donor engraftment at 24 weeks had detectable, low level (<5%) engraftment at later time points. All patients with follow up of >36 weeks show the production of recent thymic emigrants and/or de novo production of naïve T and/or B cells. In addition to improved lymphocyte values, patients have demonstrated clinical improvement including resolution of chronic diarrhea, significant weight gain, and reduced IVIG requirements.
Conclusion: This study is the first demonstration of HSC engraftment following monoclonal antibody-based conditioning of patients without chemo(radio)therapy. Specifically, this first-in-human HCT trial shows that an anti-CD117 antibody safely clears HSC niches and facilitates donor HSPC engraftment in patients with SCID. Clinical benefit has been observed with minimal to no toxicity. Four of six evaluable patients have sustained evidence of donor myeloid engraftment along with T and B lymphopoiesis, indicative of engraftment of multipotent HSC. These results suggest that antibody conditioning for HCT may be preferable to traditional chemo(radio)therapy conditioning, especially in patients with non-malignant diseases and/or increased risk of toxicities due to such agents, such as certain forms of SCID, Fanconi anemia and sickle cell disease. Anti-CD117 antibody conditioning may also be applicable to gene therapy with genetically corrected autologous HSC. The AMG 191 study is actively enrolling previously transplanted SCID patients and newly diagnosed SCID patients.
Dvorak:Alexion Inc: Consultancy; Jazz Pharmaceuticals: Consultancy. Prohaska:Forty Seven Inc: Equity Ownership, Patents & Royalties. Weissman:Forty Seven Inc.: Consultancy, Equity Ownership, Patents & Royalties. Cowan:Rocket Pharma: Consultancy; Homology Medicine: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; bluebird bio: Consultancy; California Institute Of Regenerative Medicine: Research Funding; UpToDate: Honoraria; Leadiant: Consultancy; NIH NIAD: Research Funding. Logan:Kadmon: Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite: Research Funding; TeneoBio: Consultancy; Novartis: Consultancy; Astellas: Research Funding; Abbvie: Consultancy; Incyte: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; Kiadis: Consultancy; Jazz: Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees. Weinberg:U.S. Patent Office: Patents & Royalties: patent pending - submitted for aldehyde dehydrogenase 2 (ALDH2) activators to expand hematopoietic stem cells. Shizuru:Forty Seven Inc: Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal