Introduction
Venous thromboembolic events (VTE) are a frequent complication of patients with sickle cell disease (SCD). The estimated incidence of thrombosis is 25% and is associated with increased morbidity and mortality. Recent studies have implicated leukocytes and their subsets in development and propagation of VTE in malignancies and inflammatory diseases. However the role of leukocytes have not been elucidated in SCD. We conducted a retrospective study to precisely study the association of leukocytes and their subsets in VTE.
Methods
We accessed the medical records of patients with SCD who presented to University Hospitals Seidman Cancer Center from 2004 to 2018. Information collected included demographic characteristics, comorbidities, laboratory parameters as well as treatment details. Patient records were reviewed for VTE with radiographic confirmation. Cases were defined as patients with SCD with diagnosed VTE and controls were SCD patients without VTE. Cases and controls were matched for age, gender, red blood cell (RBC) genotype and treatment. We included twice as many controls as cases for the study. Inclusion criteria were age greater than 18 years and were followed up at the outpatient sickle cell clinic. Patients were excluded if they had infection, thrombophilia, neoplasm, pregnancy or arterial thrombosis. For cases, lab parameters at the time of the VTE were recorded. Since most patients with VTE were diagnosed during an admission, lab parameters for controls were also recorded during an admission for pain crises. For both cases and controls, steady state lab data 3 months prior to their admission was also recorded and compared. Data was analyzed using STATA SE 15 version. Mann-Whitney U test was used to analyze continuous variables and Fischer's exact test was used to analyze discrete variables. P-values less than 0.05 were considered significant.
Results
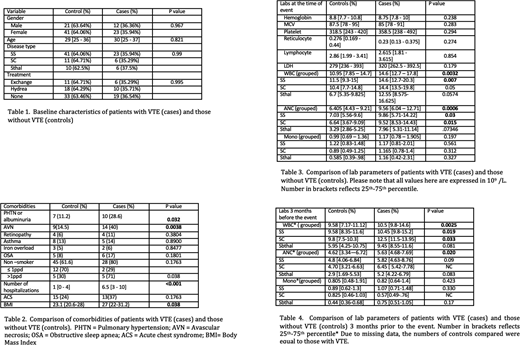
A total of 263 records were reviewed of which 97 patients were eligible for the study. There were 35 patients with VTE and 62 patients without VTE. Baseline characteristics are presented in Table 1. Compared to controls, patients with VTE had more comorbidities such as avascular necrosis (AVN; p = 0.0038), pulmonary hypertension or albuminuria (pHTN; p= 0.032), higher body mass index (BMI) (p = 0.038) and had more hospitalizations (p < 0.001). Due to small number of patients, the comorbidities were analyzed as a group across all genotypes. A complete list of comparison of comorbidities is summarized in Table 2. When compared to controls, patients with VTE as a group had higher total white blood cell count (WBC) and neutrophil count (p=0.0032 and p = 0.0006 respectively) at the time of event. When RBC genotype was taken into account, patients with SC and SS genotype had a significantly elevated neutrophil count whereas only patients with SS genotype had a higher WBC count compared to controls (Please refer to Table 3). When compared to controls, cases as a group also had WBC and neutrophil count 3 months prior to the event (p=0.025 and p=0.020 respectively). Patients with SS and SC patients had a significantly higher WBC count 3 months prior compared to their non VTE counterparts (Please refer to Table 4) No other comorbidity or lab parameter was found to be significantly different between cases and controls. A multivariate analysis could not be performed due to small study numbers.
Conclusion
Our study is the first to show that VTE in SCD was associated with higher neutrophil counts 3 months prior to the event suggesting that patients with baseline or steady state leukocytosis and neutrophilia are at increased risk of thrombosis. Larger studies are needed to confirm this association. In addition, patients with VTE also had higher incidence of comorbidities such as AVN, pHTN or albuminuria and increased hospitalizations. Given the small number of patient, a multivariate analysis was not possible. While the association between pHTN and VTE is well documented; the association of AVN and higher BMI with VTE described here is novel and needs further exploration with larger studies.
Little:Hemex Health, Inc.: Patents & Royalties; GBT: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal