Introduction: Pain is the most common complication of sickle cell disease (SCD) and a frequent cause of acute care utilization. It can be acute due to vaso-occlusive crisis or chronic with over 50% of adults experiencing chronic pain.1 Having chronic pain was associated with both high daily opioid use (≥ 90 MME) and worse quality of life (3,4). High daily opioid use in SCD was also associated with higher acute care utilization5 and ~50% of SCD adults presenting to a large urban acute-care centre were on chronic daily opioids.2 Opioids have been the mainstay of treatment for both acute and chronic pain in SCD however there is emerging evidence of the poor efficacy and high toxicity/morbidity associated with continuous daily use of opioids in any patient. These adverse effects include tolerance, dependency, opioid induced hyperalgesia, worsening of mood disorders, constipation and opioid withdrawal. The current opioid crisis demands that we identify alternatives to reduce daily high-dose opioids for treating SCD chronic pain that may also reduce acute care utilization and quality of life. Buprenorphine is a partial Mu receptor agonist and Kappa receptor antagonist. This product is approved to treat chronic pain. Naloxone is a Mu receptor antagonist. Buprenorphine/naloxone (B/N) combination products have been used to treat opioid use disorders and is increasingly used off-label to treat chronic pain in patients with opioid dependence. There is currently no published data on the use of buprenorphine alone or B/N in the management of chronic pain in adults with SCD.
METHODS: We hypothesized that use of B/N in adults with SCD reduce both acute care utilization and daily opioid use while improving overall clinical outcomes. Using a retrospective chart audit we evaluated patients transitioned to B/N between January 1st, 2016 and April 30th, 2018 who had at least six months of follow up post transition. Patients were expected to follow up in clinic at least weekly for the first 4 visits, biweekly for 4 visits, and then monthly with a SCD provider and psychotherapist. We evaluated Morphine Equivalent Daily Dose (MEDD), acute care (ED) visits, inpatient (IP) admissions, length of stay (LOS), comorbid psychiatric diagnosis, hemoglobin S% (HbS) and F% (HbF) as markers of adherence to disease modifying therapy use and serum ferritin. MEDD was calculated by averaging monthly opioid use and included both outpatient prescriptions from the state's-controlled substance database and inpatient opioid doses administered from the electronic medical record, respectively. ED/IP visits (normalized on an annual basis), average LOS, and lab values were compared over two time-periods: from the date of entry into our SCD program to the date of initiation of B/N (switch date), and from the switch date to the last follow-up date. MEDD was compared for the 6 months pre- and post- initiation of B/N. Outcomes were analysed using generalized linear mixed models with the pre- and post-switch date as the fixed factor in the model. A random effect for subject was included to account the repeated observations.
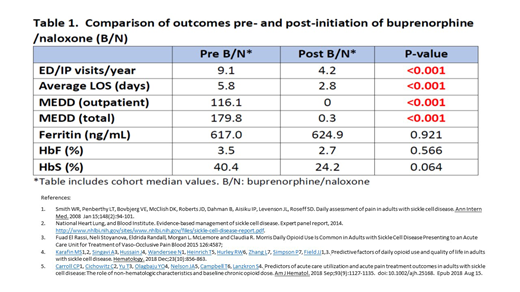
Results: Twenty-four patients were evalauted: 67% Female; median age 36 years (range 18 - 65). Genotype distribution: HbSS 15 (63%); HbSC 5 (21%); SB+Thal 4 (17%); 92% had a comorbid psychiatric condition (major depression, bipolar disorder, generalized anxiety disorder). Our results (summarized in Table 1) demonstrate significant reductions in acute care utilization (ED/IP visits, average IP LOS) and MEDD after initiation of B/N (p<0.001). No significant differences were noted in Ferritin level and HbF% between pre- and post-initiation of B/N. There was a trend towards reduced HbS% as a marker of adherence to monthly erythrocytapheresis however sample size was small.
Conclusion: These results demonstrate that Buprenorphine/naloxone is effective in reducing acute care utilization among adults with SCD and drastically lowering the amount of daily opioids used to manage chronic pain. The impact of B/N on acute care utilization and daily opioid use was independent of adherence to disease modifying therapy (hydroxycarbamide and chronic erythrocytapheresis).
Osunkwo:Micella Biopharma: Other: DSMB Member ; Terumo: Speakers Bureau; Pfizer: Consultancy; Novartis: Consultancy, Speakers Bureau. Symanowski:Boston Biomedical: Membership on an entity's Board of Directors or advisory committees; Eli Lilly: Membership on an entity's Board of Directors or advisory committees; Immatics: Membership on an entity's Board of Directors or advisory committees; Carsgen Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal