Background: Isocitrate dehydrogenase 2 (IDH2) mutations occur in 5-10% of patients (pts) with myelodysplastic syndrome (MDS) and are frequently associated with intermediate-risk cytogenetics, excess bone marrow blasts, neutropenia and sustained platelets. Enasidenib (ENA) is a selective oral inhibitor of the mutant IDH2 enzyme with single-agent activity in relapsed/refractory acute myeloid leukemia (AML). This study was designed to evaluate the efficacy and tolerability of ENA alone and in combination with azacitidine (AZA) in pts with high-risk IDH2-mutated MDS.
Methods: This is a multicenter Phase II trial for pts with IDH2-mutated MDS, AML with 20-30% marrow blasts, or chronic myelomonocytic leukemia. The study includes two cohorts: HMA-naïve pts with high-risk MDS (IPSS int-2 or high-risk; IPSS-R high-risk or very high risk; or high-risk molecular features including TP53, ASXL1, EZH2 and/or RUNX1 mutations) (Arm A) receive AZA + ENA; pts relapsed/refractory with prior HMA therapy (Arm B) receive ENA alone. All pts receive ENA at a dose of 100 mg orally daily, on days 1-28 of each 28-day cycle. In Arm A, ENA is given in combination with AZA 75 mg/m2 IV or SC on days 1-7 of each cycle. The primary efficacy endpoint is overall response rate (ORR), including complete remission (CR), marrow CR (mCR), partial remission (PR) and hematologic improvement (HI) based on the Modified International Working group (IWG) Response Criteria for MDS. The primary safety endpoint is the incidence and severity of adverse events using the Common Toxicity Criteria for Adverse Events v4.0.
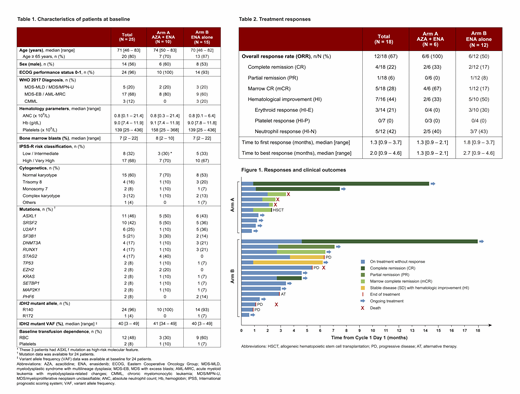
Results: Using a pre-specified data cutoff of July 1st 2019, 25 pts have been enrolled with a median follow-up of 6.4 months (range, 2.4 - 17.1); 10 HMA-naïve pts (Arm A) and 15 HMA-failure pts (Arm B). The median age was 71 years (range, 46-83) (Table 1). Sixteen pts (64%) had neutropenia (absolute neutrophil count < 1.0 x 109/L) and 20 pts (80%) had anemia (hemoglobin < 11 g/dL), including 12 (48%) red blood cell (RBC) transfusion-dependent (TD) pts at baseline. Seventeen pts (68%) had high or very high risk IPSS-R. Nineteen pts (76%) had diploid or +8 cytogenetics, and 5 pts (20%) had -7 or complex karyotype. High-risk co-occurring mutations included ASXL1 (46%), RUNX1 (17%), EZH2 (8%) and TP53 (8%).
Among 18 evaluable pts (7 too early for response assessment), the ORR was 67% (12/18) (Table 2). In HMA-naïve pts, 6/6 (100%) responded to therapy, including 2 CRs and 4 mCRs (1 with HI for neutrophils [HI-N]). In HMA-failure pts, 6/12 (50%) responded, including 2 CRs, 1 PR, 1 mCR (with HI-N) and 2 with stable disease with HI (1 with HI-N, 1 with HI erythroid). Interestingly, 3 pts who achieved CR also had clearance of the IDH2 mutation (1 in Arm A; 2 in Arm B). Two of 5 pts (40%) and 3/7 pts (43%) with neutropenia at baseline achieved HI-N in Arm A and B, respectively. Median time to first and best response were both 1.3 months (range, 0.9-2.1) in Arm A and 1.8 months (range, 0.9-3.7) and 2.7 months (0.9-4.6), respectively in Arm B. Among evaluable pts with RBC TD at baseline, 0/2 pt and 3/8 (38%) pts achieved transfusion independence, in Arm A and B, respectively. At last follow-up, 16 pts remain on treatment (7 with ongoing response) and 9 pts stopped treatment: 4 due to progression, 1 pt decision, 1 underwent allogeneic transplant and 3 responding pts with mCR died from pneumonia or other infectious complications while on study (Figure 1).
Adverse events (AEs) of any grade were reported in 17/25 pts (68%) and grade 3-4 AEs were reported in 11/25 pts (44%). Most AEs were manageable without dose interruption. The most common non-hematological AEs were unconjugated hyperbilirubinemia (39%), nausea (33%), fatigue (33%), pneumonia (22%) and diarrhea (17%). Possible differentiation syndrome (DS) was reported in 3 pts on days 31, 38 and 42 of treatment; 2 pts received dexamethasone with resolution, and 1 pt required hydrea and was ultimately determined to have progression to AML. Four pts developed leukocytosis (white blood cell count of 15.3, 28.3, 35.7, 56.6 x 109/L), with 3 at the time of possible DS and 1 at day 119 considered unrelated to DS.
Conclusion: Enasidenib is well tolerated and shows promising efficacy in IDH2-mutated high-risk MDS. The ORR was 67%, including 100% in newly diagnosed pts receiving the combination of azacitidine plus enasidenib and 50% ORR in HMA-failure pts receiving enasidenib alone. The study continues to accrue and updated results will be presented at ASH.
DeZern:Celgene: Consultancy; Astex Pharmaceuticals, Inc.: Consultancy. Takahashi:Symbio Pharmaceuticals: Consultancy. Konopleva:Astra Zeneca: Research Funding; Calithera: Research Funding; Ablynx: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Kisoji: Consultancy, Honoraria; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Ascentage: Research Funding; Genentech: Honoraria, Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Forty-Seven: Consultancy, Honoraria; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Agios: Research Funding. Loghavi:MDACC: Employment; GLG Consultants: Consultancy; AlphaSights: Consultancy. Alvarado:Jazz Pharmaceuticals: Research Funding; Abbott: Honoraria. Ravandi:Selvita: Research Funding; Xencor: Consultancy, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cyclacel LTD: Research Funding; Macrogenix: Consultancy, Research Funding; Menarini Ricerche: Research Funding. Sasaki:Otsuka: Honoraria; Pfizer: Consultancy. Sekeres:Celgene: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees. Nazha:Abbvie: Consultancy; Incyte: Speakers Bureau; Daiichi Sankyo: Consultancy; MEI: Other: Data monitoring Committee; Novartis: Speakers Bureau; Jazz Pharmacutical: Research Funding; Tolero, Karyopharma: Honoraria. Roboz:Trovagene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Actinium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amphivena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astex: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eisai: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees; Otsuka: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kantarjian:Immunogen: Research Funding; Cyclacel: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Jazz Pharma: Research Funding; Astex: Research Funding; Ariad: Research Funding; Amgen: Honoraria, Research Funding; BMS: Research Funding; Takeda: Honoraria; Agios: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Research Funding; Daiichi-Sankyo: Research Funding. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. DiNardo:medimmune: Honoraria; abbvie: Consultancy, Honoraria; jazz: Honoraria; syros: Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; agios: Consultancy, Honoraria; celgene: Consultancy, Honoraria; daiichi sankyo: Honoraria.
Enasidenib is not approved for the treatment of myelodysplastic syndrome
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal