Background: Pacritinib (PAC), an oral JAK2/IRAK 1 inhibitor, has demonstrated clinical benefit in myelofibrosis (MF) patients in two prior Phase 3 studies (PERSIST-1, PERSIST-2). PAC203 is a randomized Phase 2 dose-finding study in patients with MF who were intolerant of or failed to benefit from ruxolitinib. Herein, we describe the results, including pharmacokinetic and pharmacodynamic (PK/PD) analyses, used to identify the recommended dose of PAC for further study.
Methods: Patients with DIPSS intermediate-1, -2, or high-risk MF who were intolerant of ruxolitinib (treatment ≥28 days complicated by development of RBC transfusion requirement or grade ≥3 anemia or thrombocytopenia, hematoma, and/or hemorrhage while treated with <20 mg BID) or who had failed to benefit from ruxolitinib (treatment ≥3 months with <10% spleen volume reduction or <30% decrease in spleen length or regrowth to these parameters) were randomized 1:1:1 to PAC 200 mg BID, 100 mg BID, or 100 mg QD stratified by baseline platelet count. Efficacy endpoints included the proportion of patients with ≥35% spleen volume reduction (SVR) and the proportion with ≥50% total symptom score (TSS) reduction. Results from PAC203 were pooled with an integrated data set from prior studies for population PK (prior Phase 1-3 studies) and PK/PD (prior Phase 3 studies) analysis to determine the recommended dose of PAC. Data cut was 01-April-2019.
Results: PAC203 included 164 patients; 161 received treatment. Median age was 69 years; 43% had platelet count <50,000/mL and 71% had hemoglobin <10 g/dL. In 105 patients with molecular data, driver mutations were present in 99 (94%), with CALR detected in only 9 (8.6%). High-risk molecular type (ASXL1, EZH2, SRSF2, IDH1/2, or U2AF1 Q157) was present in 41 (39%); an additional 5 (4.8%) without such mutations had a mutation in TP53. Patients had been treated with ruxolitinib for a median of 1.4 (range: 0.6-3.0) years; 68% were intolerant to and 73% failed to benefit from ruxolitinib, while 47% met both criteria.
The most common treatment-emergent non-hematologic adverse events (AEs) were gastrointestinal, including diarrhea (20.5%; Grade 3: 3.1%) and nausea (20%; Grade 3: 0.6%), distributed similarly across arms. The most common hematologic AEs were thrombocytopenia and anemia, both occurring at higher frequencies at 200 mg BID (32% and 22% respectively); this did not, however, lead to higher rates of Grade 3/4 hemorrhage at higher doses (200 mg BID: 5.6%; 100 mg BID: 0%; 100 mg QD: 5.8%; all Grade 3). Similarly, the highest dose saw no excess in Grade 3/4 cardiac (200 mg BID: 3.7%; 100 mg BID: 7.3%; 100 mg QD: 3.7%; all Grade 3) or infectious (200 mg BID: 15%; 100 mg BID: 11%; 100 mg QD: 12%) AEs. In this cohort of advanced MF patients, there were 7 Grade 5 (fatal) AEs: 2 at 200 mg BID (sepsis, subdural hematoma), 3 at 100 mg BID (disease progression, subdural hematoma, heart failure), and 2 at 100 mg QD (sepsis, tuberculosis).
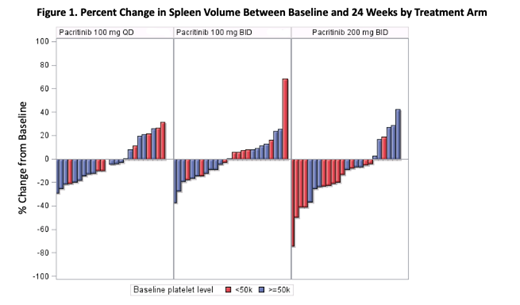
The 200 mg BID arm had the highest observed rates of SVR ≥35% (200 mg BID: 9.3%; 100 mg BID: 1.8%; 100 mg QD: 0.0%; Figure 1) and TSS ≥50% (200 mg BID: 7.4%; 100 mg BID: 5.5%; 100 mg QD: 5.8%). Of the 5 patients with SVR ≥35% at the 200 mg BID dose, 4 had platelet counts <50,000/mL, representing a 17% (4/24) response rate in this population. PK/PD modeling based on 258 patients demonstrated that that the expected improvement in spleen volume and TSS at 200 mg BID was substantially greater than that projected with the 100 mg BID and 100 mg QD regimens, and that higher exposure quartiles were associated with increased probability of spleen volume reduction. On logistic regression, previous use of ruxolitinib was significantly associated with less spleen volume reduction, but not with TSS reduction.
Conclusion: PAC 200 mg BID is generally well tolerated and has demonstrated clinical activity, particularly in patients with severe thrombocytopenia (platelet count <50,000/mL). The PAC203 population is characterized by high-risk, advanced, and heavily pre-treated disease; even in this group, PAC 200 mg BID was associated with reduction in spleen size and symptom burden using stringent, front-line response criteria (SVR ≥35% and TSS ≥50%). An upcoming phase 3 study (PACIFICA), including patients with MF and severe thrombocytopenia that are either naïve to or have had a limited duration of prior JAK2 inhibitor therapy, will compare PAC 200 mg BID vs. physician's choice.
Gerds:Celgene Corporation: Consultancy, Research Funding; CTI Biopharma: Consultancy, Research Funding; Pfizer: Consultancy; Sierra Oncology: Research Funding; Incyte: Consultancy, Research Funding; Roche: Research Funding; Imago Biosciences: Research Funding. Savona:AbbVie: Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Patents & Royalties; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Sunesis: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Selvita: Membership on an entity's Board of Directors or advisory committees. Scott:Celgene Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Agios: Speakers Bureau. Talpaz:Imago BioSciences: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; CTI BioPharma: Research Funding; Samus Therapeutics: Research Funding; Novartis: Research Funding; Incyte: Research Funding; Constellation: Research Funding. Harrison:Incyte: Speakers Bureau; Shire: Speakers Bureau; Gilead: Speakers Bureau; Promedior: Honoraria. Yacoub:Incyte: Consultancy, Speakers Bureau. Vannucchi:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Italfarmaco: Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Mead:Bristol Myers-Squibb: Consultancy; Novartis: Consultancy, Honoraria, Other: Travel/accommodation expenses, Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding; CTI: Honoraria, Research Funding; Pfizer: Consultancy. Buckley:CTI BioPharma: Employment, Equity Ownership. Mould:CTI BioPharma: Consultancy. Tyavanagimatt:CTI BioPharma: Employment, Equity Ownership. Smith:CTI BioPharma: Employment, Equity Ownership. Mascarenhas:Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmaessentia: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Roche: Consultancy, Research Funding; Merck: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Research Funding; Promedior: Research Funding; Merus: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal