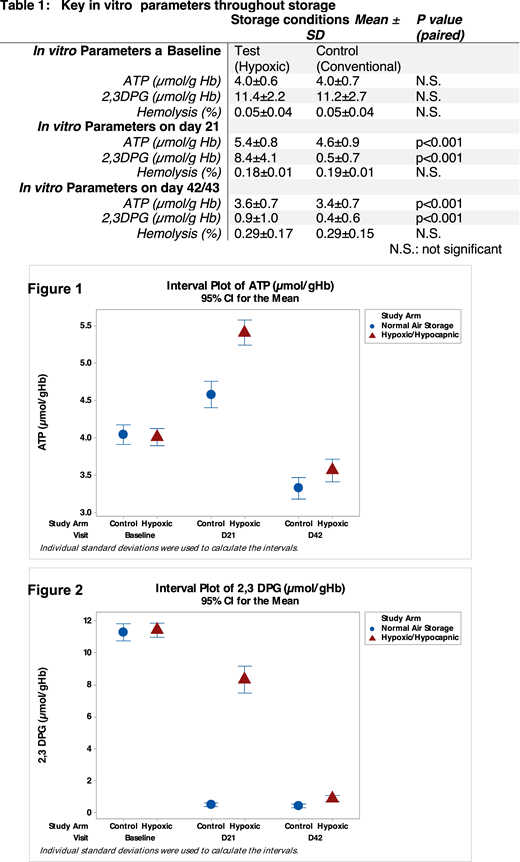
Oxidative damage, dysmetabolism and decreased levels of 2, 3-diphosphoglycerate (2,3-DPG) affect red blood cell (RBC) survival and the affinity of Hemoglobin for oxygen are relevant hallmarks of RBC storage lesion, especially in specific clinical contexts. We hypothesized that oxygen and carbon dioxide reduction followed by hypoxic/hypocapnic storage would result in amelioration of RBC lesion assessed at the end of their shelf-life and at the shelf-life mode (21 days).To determine the relevance of hypoxic/hypocapnic storage in humans, a pivotal, prospective, randomized, two‐arm, crossover, three‐center trial was conducted to evaluate [Hemanext®] hypoxic/hypocapnic processing system and was used to assess whether this process and storage method met standard FDA acceptability criteria for long-term RBC storage. The Test arm consisted of whole blood (WB)‐derived, leukoreduced RBC in AS-3 additive processed at room temperature with the Hemanext system for 3 hours to achieve hypoxic/hypocapnic state within 12 hours of phlebotomy which was maintained hypoxic during storage for up to 43 days at 1‐6°C (Test).Unprocessed units (Control) were stored within 8 hours under conventional storage conditions. Subjects (N=100) donated CP2D WB (500 ± 50 mL) and a minimum of 93 pairs of RBC units per arm were analyzed for in vitroquality parameters. For in vivo analysis at end of storage, RBCs from 19 test subjects and 21 control subjects (14 paired) from two sites were radiolabeled with 51‐Cr/99‐Tc(m), autologously transfused, and analyzed for 24-hour recovery. Differences between the Test and Control groups were analyzed using the paired t-test (Wilcoxon test where necessary). Paired analyses of 24-hour in vivorecoveries on day 43 was 89.3±4.5% and 84.8±6.2% for the test and control, respectively; p<0.01). Significantly higher levels of 2,3-DPG and adenosine 5'-triphosphate (ATP) were maintained for the Test group by days 21 and 42/43 of storage (Table 1). Percentage of hemolysis was similar in both groups. In summary, these data demonstrate that RBCs preserved in a user-friendly, self-contained hypoxic storage system are superior than the conventionally stored RBCs and may provide more viable RBCs for transfusion at 6 weeks of storage.
Disclaimer: The51‐Cr/99‐Tc(m)labeling at one of the three sites occurred without prior RDRC approval and did not meet GMP standards.
Cancelas:Fresenius-Kabi: Research Funding; Cerus Co.: Research Funding; TerumoBCT: Consultancy, Research Funding; Hemanext: Consultancy, Research Funding; Macopharma Inc: Research Funding; Cytosorbents: Research Funding; Cellphire: Research Funding; Velico: Consultancy, Research Funding. Yoshida:New Health Sciences Inc.: Employment, Equity Ownership. Dioguardi:Hemanext: Employment. Iselin:Hemanext: Employment. Dunham:Hemanext: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal