Storage lesion accumulates during refrigerated storage of RBC, and development of novel methods and/or additive solutions are needed for more efficacious RBC with reduced burden of harmful byproducts. Autologous 24-hr post-transfusion recovery (PTR24) at the end of storage measured in healthy volunteers is the only in vivo practical metrics to evaluate consequences of RBC storage. PTR24 studies are logistically and economically demanding and as such, the identification of easier biomarkers of stored blood quality can expedite the development and testing of novel storage strategies.
We performed a prospective, randomized, dual‐arm, crossover, two‐center radio labeled 24-hr PTR24 study was conducted as a part of the pivotal study to evaluate Hemanext® hypoxic/hypocapnic processing system for meeting standard FDA acceptability criteria for RBC storage. Subjects donated CP2D whole blood (500 ± 50 mL). The Test arm consisted of whole blood‐derived RBC, leukoreduced, and processed to achieve hypoxic/hypocapnic state in AS-3 additive within 12 hours of phlebotomy then stored for 42 days at 1‐6°C under hypoxic conditions (Test). Unprocessed normal atmosphere stored units (Control) were stored within 8 hours under conventional storage condition. PTR24 data was obtained from 19 test subjects and 21 control subjects (14 paired) by infusion of 51‐Cr/99‐Tc(m) radiolabeled RBCs*. At day 0, 21 and 42, in vitro metrics, including conventional quality parameters (%hemolysis, ATP, 2,3-DPG, morphology, microparticles and deformability), as well as a large panel of metabolites and lipids were analyzed for correlations with PTR24. Bioactive lipids were quantified by liquid chromatography-tandem mass spectrometry with multiple reaction monitoring (LC-MS/MS-MRM).
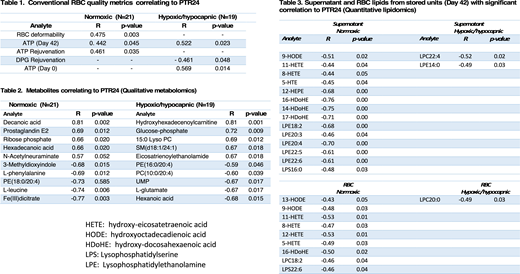
Pearson's correlation coefficients for PTR24 and analytes were examined; correlation pattern differed significantly between normal air Control and hypoxic Test RBCs and thus analyzed separately. Among the conventional RBC quality metrics, ATP as well as ability to rejuvenate ATP and 2,3-DPG at end of storage correlated with PTR24. RBC deformability correlated only with Control, while reduced oxidative damage in Test removed its dependency. Test showed stronger correlations with ATP and other metabolic parameters. 302 metabolites and lipids were analyzed by qualitative metabolomics workflow, and significant correlations were observed with |R| ≥ 0.5 for 37 compounds in normal air stored RBCs (15 with p ≤ 0.05), and 41 compounds in hypoxic RBCs (19 with p ≤ 0.05). The top 5 correlating and inversely correlating compounds for each condition are reported along with p-values in Table 1. For RBC stored in normal air, strong correlations were primarily seen with membrane lipids and associated fatty acids, amino acids, and the Pentose Phosphate Pathway intermediates. Hypoxic storage PTR24 values primarily correlated with alternative membrane components, amino acids, and Pentose Phosphate Pathway intermediates (Table 2). Among 67 bio-reactive lipids analyzed quantitatively, negative correlations of oxylipins (9- or 13-HODEs, 5-, 8-, 11-, 12-HETEs) to PTR24 were observed under normal conditions, but not under hypoxic condition (Table 3).
In summary, RBC deformability correlated only with PTR24 of normal air stored RBC, while reduced oxidative damage in hypoxic/hypocapnic conditions removed this dependency. Although normal air and hypoxic stored RBCs show differing metabolite correlates with PTR24, significant associations tend to occur within similar overall pathways. Comparing the PTR24-metabolite correlations between normal air and hypoxic/hypocapnic RBC storage reveals that intermediates of oxidative stress management and membrane homeostasis are potential predictors of post-transfusion recovery. Detailed analysis of lipids supports that hypoxic/hypocapnic storage may reduce membrane lipid degradation and generates the hypothesis that the metabolism of bioactive lipids under hypoxia/hypocapnia may be different from the one found in RBC stored under normal air storage conditions.
* Radio-labeling for 14 subjects occurred without prior RDRC approval; one site did not meet GMP standards.
Yoshida:New Health Sciences Inc.: Employment, Equity Ownership. D'Alessandro:Hemanext, Inc.: Membership on an entity's Board of Directors or advisory committees; Omix Technologies, Inc.: Other: Founder. Nemkov:Omix Technologies, Inc.: Equity Ownership, Other: Founder. Dioguardi:Hemanext: Employment. Iselin:Hemanext: Employment. Dunham:Hemanext: Employment. Cancelas:Hemanext: Consultancy, Research Funding; Fresenius-Kabi: Research Funding; Cerus Co.: Research Funding; TerumoBCT: Consultancy, Research Funding; Macopharma Inc: Research Funding; Cytosorbents: Research Funding; Cellphire: Research Funding; Velico: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal