CD6 is a co-stimulatory receptor expressed on T cells that binds activated leukocyte cell adhesion molecule (ALCAM), a ligand expressed on antigen presenting cells and various epithelial and endothelial tissues. The CD6-ALCAM pathway plays an integral role in modulating T cell activation, proliferation and trafficking and is central to inflammation. Early studies by Soiffer et al. demonstrated that ex vivo depletion of CD6+ donor cells prior to hematopoietic cell transplantation (HCT) decreased the incidence of acute graft versus host disease (aGVHD), highlighting the importance of CD6+ cells in GVHD pathogenesis. Itolizumab, a humanized anti-CD6 monoclonal antibody, has been shown to modulate T cell activation and proliferation. The aim of this study was to characterize: (1) expression of CD6 and ALCAM, and (2) activity of itolizumab on T cell responses in peripheral blood from HCT patients pre- and post-aGvHD.
We analyzed immune reconstitution in 31 adult patients who underwent HLA matched donor HCT for hematological malignancies. Patients received peripheral blood stem cell grafts and GVHD prophylaxis with tacrolimus and methotrexate. Twelve of 31 patients developed aGVHD at a median of 58 days, range 27-208, after HCT and systemic treatment was started in 83% of these cases. aGVHD grade severity was 25%, 58.3% and 16.7% of grade I, II and IV, respectively. Patient samples were collected at 1, 2 and 3 months after HCT and analyzed using multi-color flow cytometry. Nine healthy donors (HD) were analyzed as controls. Suppressive activity of itolizumab was tested using peripheral blood mononuclear cells (PBMC) obtained from HD and patients before (preGVHD) and after (postGVHD) aGvHD onset (within 30 days). PBMC were stimulated with antiCD3/CD2/CD28 coated beads in the presence of itolizumab or isotype control (cetuximab) for 72 hours. T cell proliferation was measured by CFSE dilution, while T cell activation and maturation was measured by expression of CD25 and CD45RO, respectively. For statistical analysis, non-parametric unpaired (Mann-Whitney) or paired (Wilcoxon matched-pairs signed rank) test were used.
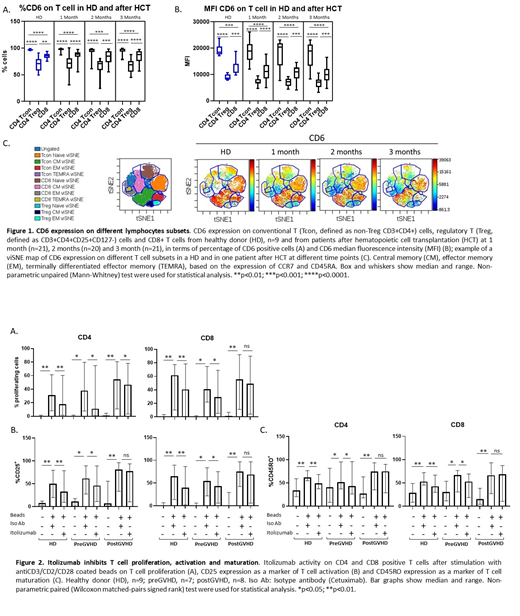
CD6+ T cells reconstituted early after transplant, accounting for 95% of positive CD3 T cells, range 57-100 at 1 month. Similar to HD PBMC, in the first 3 months after HCT, CD4 Tcon had the highest CD6 expression, while CD4 Treg had a lower CD6 expression compared to both CD4 Tcon and CD8 T cells (Fig 1A and 1B). To characterize the expression of CD6 on different T cell subsets, we used a t-Distributed Stochastic Neighbor Embedding (t-SNE) algorithm and visualized the data using a viSNE map (Fig 1C). Within the Tcon compartment, there were no differences in expression of CD6 between HD and patients at all 3 time points. Within CD4 Treg and CD8 T cells, CD6 expression was reduced in naïve CD8 T cells and CM Treg after transplant compared to HD. In HD, ALCAM expression was detected in 35% of CD14+ monocytes, 23% of CD19+ B cells, 20% of myeloid (CD11c+ CD123-) DCs and 97% of plasmacytoid (CD11c-CD123+) DCs. After HCT, expression of ALCAM in DC compartments was similar to HD.
In functional studies, itolizumab inhibited CD4 and CD8 T cell proliferation in preGVHD samples, similar to HD controls. This effect was less prominent in samples collected from patients who had developed GVHD and were already receiving immunosuppressive medications, potentially confounding the ability to assess the effect of itolizumab in this assay (Fig 2A). Similar results were observed for CD25 (Fig 2B) and CD45RO (Fig 2C) expression pre- and post-aGVHD. Finally, itolizumab did not increase rates of cell death in samples from HCT patients as assessed by Annexin V expression, suggesting that itolizumab-mediated T cell inhibition was not due to increased T cell apoptosis. There was a slight increase in Annexin V expression in HD vs isotype control (21%, range 10-43 vs 15%, range 11-31, p= 0.0273).
In conclusion, we demonstrate for the first time that CD6+ T cells reconstitute rapidly in peripheral blood after HCT and that CD6 expression is highest in Tcon while lowest in Treg (Tcon>CD8>Treg). Itolizumab efficiently inhibits T cell proliferation and activation after in vitro TCR stimulation of PBMC from aGvHD patients, thus representing a potential therapeutic for treating aGvHD. A phase I/II study using itolizumab as first line treatment in combination with steroids for patients with aGVHD is currently ongoing (NCT03763318).
Rambaldi:Equillium: Research Funding. Koreth:Amgen: Consultancy; Cugene: Consultancy; Equillium: Consultancy. Cutler:Pharmacyclics: Consultancy; Omeros: Consultancy; Kadmon: Consultancy; BiolineRx: Other: DSMB; Cellect: Other: DSMB; Kalytera: Other: DSMB; ElsaLys: Consultancy; Genentech: Consultancy; BMS: Consultancy; Jazz: Consultancy; Incyte: Consultancy; Fate Therapeutics: Consultancy. Nikiforow:Kite/Gilead: Honoraria; Novartis: Honoraria; NKarta: Honoraria. Ho:Omeros Corporation: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Consultancy. Soiffer:Jazz: Consultancy; Gilead, Mana therapeutic, Cugene, Jazz: Consultancy; Juno, kiadis: Membership on an entity's Board of Directors or advisory committees, Other: DSMB; Cugene: Consultancy; Mana therapeutic: Consultancy; Kiadis: Other: supervisory board. Ampudia:Equillium: Employment. Ng:Equillium: Employment, Equity Ownership. Connelly:Equillium: Employment, Equity Ownership. Ritz:Equillium: Research Funding; Merck: Research Funding; Kite Pharma: Research Funding; Aleta Biotherapeutics: Consultancy; Celgene: Consultancy; Avrobio: Consultancy; LifeVault Bio: Consultancy; TScan Therapeutics: Consultancy; Talaris Therapeutics: Consultancy; Draper Labs: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal