Post-transplant cyclophosphamide (PTCy) in combination with tacrolimus and mycophenolate mofetil (MMF) has been used increasingly in recent years to prevent graft-versus-host disease (GvHD) in patients undergoing hematopoietic cell transplantation (HCT), and has been proven safe and effective regardless of HLA matching criteria. Historically, the therapeutic dose level recommended for tacrolimus has ranged from 10 to 15 ng/mL, when combined with methotrexate, and between 5-10 ng/mL when combined with sirolimus. However, data on the optimal starting dose and serum level of tacrolimus when combined with PTCy does not exist. Given tacrolimus's broad inhibition on T-cells activation and the PTCy's selective inhibition on alloreactive T-cells, we hypothesized that lower serum levels of tacrolimus could suffice to achieve optimal transplant outcomes.
We retrospectively identified a consecutive case series of 219 patients who received HCT with PTCy (50 mg/kg on days +3 and +4) in combination with tacrolimus and MMF (day +5) as GvHD prophylaxis at City of Hope from January 2011 to June 2018. Tacrolimus was delivered with continuous intravenous infusion until engraftment, then switched to equivalent oral dose. Tacrolimus dosing was weight-based (WBD) in 80 patients and fixed dose (FD) of 1 mg in 139 patients. We captured Tacrolimus levels at two different time points: 1. the initial steady state (ISS) and 2. at engraftment level (EL) to identify which time point will correlate with better HCT outcomes. At each time point, transplant outcomes were compared between patients with serum levels ≥10 ng/mL and <10 ng/mL. We analyzed and compared the overall survival (OS), progression-free survival (PFS), relapse rate, non-relapse mortality (NRM), cumulative incidence of Grade 2-4 acute GVHD and chronic GVHD between the two groups by univariate and multivariate analysis. P values were 2 sided at a significance level of 0.05. Correlation between method of administration and serum level of tacrolimus was examined by chi-square test.
Patients received HCT either from a haploidentical (n=175), matched (n=6), or mismatched donor (n= 38). Tacrolimus levels at the ISS (median: day +9 of HCT, range 8-16) was <10 ng/mL in 181 patients with median age of 52 years (interquartile range: 30-62) and ≥ 10 ng/mL in 38 patients with median age of 39 years (interquartile range: 18-55), (P = 0.004). Regardless of the conditioning regimen, tacrolimus level at ISS was <10 ng/ml in majority of patients (81% in myeloablative conditioning and 84 in reduced intensity conditioning). Majority of patients (91%) who received peripheral blood stem cells as graft source were more likely to have tacrolimus levels <10 ng/ml, whereas 56% of patients who received bone marrow as graft source had tacrolimus levels <10 ng/ml. Lastly, majority of patients (89%) who received tacrolimus at the FD had a serum level of <10 ng/mL at ISS as compared to 73% of patients who received the drug at WBD of the drug (P=0.003)
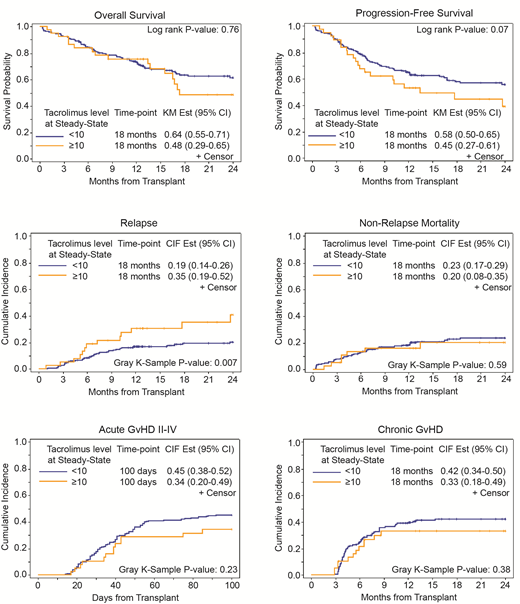
At ISS, 18 months OS, PFS, relapse rate were 64%, 58% and 19%, respectively, in patients with tacrolimus level <10 ng/mL, which compared favorably to 48%, 45%, and 35%, respectively, in patients with Tacrolimus level ≥10 ng/mL (Figure 1). In multivariable analysis, patients with tacrolimus level <10 ng/mL had lower relapse rate (HR= 0.53; 95% CI: 0.26-1.05 p=0.067) and better PFS (HR=0.50; 95% CI: 0.31-0.82, P= 0.006). While no difference was seen in OS (p= 0.76), cumulative incidence of NRM (P=0.59), acute GVHD grade II-IV (P=0.23), grade III-IV (P=0.46) or chronic GVHD (p=0.38) between the two groups at the ISS. In the multivariable analysis and after adjusting for all baseline and clinically relevant variables, EL did not correlate with any outcome
In conclusion, tacrolimus dosing at a FD was more likely resulting in ISS of 4-10 ng/mL (86% of patients in <10 ng/mL group). ISS tacrolimus levels of less than 10 ng/mL was correlated with lower relapse rate and better PFS, without a significant increase in GVHD or NRM.
Salhotra:Celgene: Other: Research Support; Kadmon Corporation: Other: Non paid consultant. Aldoss:Helocyte: Consultancy, Honoraria, Other: travel/accommodation/expenses; Jazz Pharmaceuticals: Honoraria, Other: travel/accommodation/expenses, Speakers Bureau; Agios: Consultancy, Honoraria; AUTO1: Consultancy. Stein:Amgen: Consultancy, Speakers Bureau; Celgene: Speakers Bureau; Stemline: Speakers Bureau. Nakamura:Alexion: Other: support to a lecture at a Japan Society of Transfusion/Cellular Therapy meeting ; Merck: Membership on an entity's Board of Directors or advisory committees; Celgene: Other: support for an academic seminar in a university in Japan; Kirin Kyowa: Other: support for an academic seminar in a university in Japan.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal