Introduction: Lenalidomide (Len) is increasingly used at earlier lines of treatment for patients with multiple myeloma (MM) and is usually continued until disease progression. Subsequent treatment choices are based on prior treatment, response, cytogenetics and overall performance status. The natural history of patients treated with Len is not fully captured by clinical trials due to limitations with long-term follow-up. It is also apparent that those treated in the real world have different outcomes to those treated within clinical trials. Whilst clinical trial data is emerging for Len refractory treatments, real world data for this group is limited. We therefore examined a dataset of patients treated with Len that were followed up for subsequent treatments and outcomes.
Methods: This was a retrospective cohort review from 2 UK myeloma centres of patients with relapsed MM who received Len between 2006 and 2017. Data were collected for demographics, Len treatment (T0), subsequent treatments after Len (T1, T2, T3 etc) and enrollment into clinical trials. Response was assessed by IMWG criteria and measures of survival determined by Kaplan-Meier analysis. Differences between survival were assessed by log rank test, comparison of variables between patients was performed using student t-test, Mann Whitney U or Fisher's exact test.
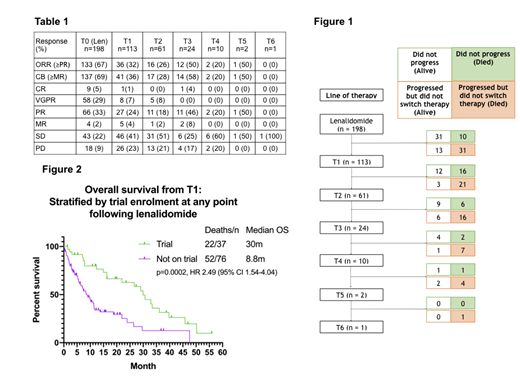
Results: 461 patients were identified between August 2006 and September 2017 of whom 263 were excluded due to incomplete data or a non-myeloma diagnosis. 198 patients (median age 66 years (35-88)) were evaluable who commenced Len a median of 41 months (0.5-210m) from diagnosis. Patients had a median of 2 prior lines (1-3); 73% had received proteasome inhibitor (PI) and thalidomide. Toxicity stopped Len use in 11 patients. There were 114 patients (72% of 158 evaluable) refractory to Len after initial response. Of these 90 (57%) had biochemical evidence of disease progression (PD) but continued on Len for a median of 3.45m (0-20.1m); 24 (26%) continued on Len for >6 months. 41 (20.7%) patients did not have PD, of whom 31 (15.7%) were alive and 10 (5%) died (Figure 1). Numbers of patients receiving subsequent treatments after Len were as follows: T1 113 (57%); T2 61 (31%); T3 24 (12%); T4 10 (5%); T5 2 (1%); T6 1 (0.5%). The mortality during each treatment line was: T1 37 (33%); T2 22 (36%); T3 9 (38%); T4 5 (50%). The most common treatment immediately after Len was pomalidomide-dexamethasone. Response rates by each subsequent treatment are shown in Table 1. Proportions of stable disease increased with subsequent lines. After a median follow-up of 33.8m from Len, the PFS for each subsequent treatment was: T0 11.1m; T1 5.7m; T2 6.6m; T3 6.7m; T4 3.6m. As the majority of patients were refractory to Len at the start of next treatment, PFS2 from Len was assessed to determine optimal salvage treatment. The median PFS2 was similar irrespective of treatment (pomalidomide (n=28) 23m, bortezomib and panobinostat (n=12) 24m, bendamustine (n=16) 25m, clinical trials (n=9) 19m, other (n=48) 25m (p=ns)). The median OS from diagnosis was 87.4m and the median OS from Len PD or change of therapy (if without PD) was 14.7m. Overall 37 (33%) enrolled into a clinical trial at any time after Len. Those treated in a clinical trial had a superior OS to those that did not (median OS 30.0m vs 8.8m, p=0.0002; HR: 2.49, Figure 2). There was a high early mortality in the non-trial group with a 6 month OS after Len PD of 63.2% (trial) vs 91.9% (non-trial) (p=0.0017, HR 3.2, 95% CI 1.5-6.7). Trial patients also had a higher median eGFR (77.5 vs 61 ml/min (p=0.02)) and better performance status (p=0.016) than those not enrolled suggesting this was a better biological population.
Conclusion: The outcomes for patients with relapsed MM following Len are poor with an OS of 14.7m and 15.7% dying before receiving further treatment. Decreasing numbers of patients receive subsequent treatments, with few able to get 5 or 6 further lines after Len. These outcomes are likely to change as Len moves to front line and immunotherapies are incorporated in routine practice. This data-set therefore serves as a baseline with which to compare such advances. The superior outcomes for those in clinical trials may in part reflect the potential of novel treatments with different modes of action in Len and PI exposed patients. However, the differences in performance status and renal function provide evidence of the disparity between trial and real world data-sets.
Lee:Autolus Therapeutics: Equity Ownership, Research Funding. Parrish:AbbVie: Consultancy, Honoraria; JAZZ: Honoraria; JAZZ: Honoraria; Takeda: Honoraria, Other: Financial support to attend scientific meetings; Takeda: Honoraria, Other: Financial support to attend scientific meetings; Celgene: Other: Financial support to attend scientific meetings; Janssen: Honoraria; AbbVie: Consultancy, Honoraria; Celgene: Other: Financial support to attend scientific meetings; Janssen: Honoraria. Yong:Amgen: Research Funding, Speakers Bureau; Autolus: Consultancy; Janssen: Speakers Bureau; Sanofi: Speakers Bureau; Takeda: Research Funding, Speakers Bureau. Cook:Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau. Popat:Takeda: Honoraria, Other: travel, accommodations, expenses; Celgene Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations, expenses; Janssen: Honoraria, Other: travel support to meetings; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal