Background and significance: Normal B-cells constantly exchange information with their environment and depend on external cues for proliferation and survival that engage multiple divergent pathways (e.g. cytokine receptors; B-cell receptor, BCR). The dependency of normal B-cells on signaling input from a diverse repertoire of surface receptors is in contrast to transforming oncogenes that engage one single pathway. Beyond the established concept that diverse signal input from multiple cell surface receptors becomes dispensable in transformed cells, we here provide evidence that inactivation of divergent pathways that are not aligned with the principal oncogenic driver represents a critical step during malignant transformation. Tracking early stages of leukemia-initiation, we identified convergence on one principal oncogenic driver and inactivation of diverging pathways as critical events during B-cell transformation. Our results support a scenario in which reactivation of divergent and potentially conflicting signaling pathways represents a powerful barrier to malignant transformation. Here we studied the interaction of STAT5- and ERK-signaling pathways during normal B-cell development and malignant B-cell transformation, and found that convergence on one principal oncogenic driver represents a critical event during B cell transformation and a previously unrecognized vulnerability.
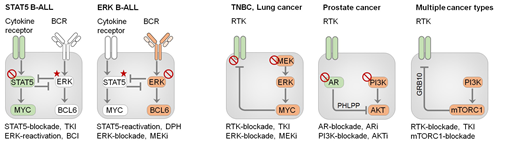
Results: Our analysis of 987 patient-derived B-cell acute lymphoblastic leukemia leukemia (B-ALL) samples revealed that individual mutations did not promote leukemogenesis unless they converged on one single oncogenic pathway. Mutations that were not aligned with the central oncogenic driver would activate divergent pathways and subvert malignant transformation. Oncogenic lesions in B-ALL frequently mimic survival and proliferation signals downstream of cytokine receptors (STAT5) or the B cell receptor (ERK). STAT5- (286 cases) and ERK- (386 cases) activating lesions were frequently found but rarely co-occurred in the same sample (35 cases; P=2.5E-16). Single-cell mutation and phosphoprotein analyses revealed that even in these rare cases, oncogenic STAT5- or ERK-activation were mutually exclusive and segregated to competing clones. STAT5 and ERK engaged opposing biochemical and transcriptional programs orchestrated by MYC and BCL6, respectively. Genetic reactivation of the divergent (suppressed) pathway came at the expense of the principal oncogenic driver and reversed malignant transformation. Conversely, Cre-mediated deletion of divergent pathway components triggered leukemia-initiation and accelerated development of fatal disease. Thus, persistence of divergent signaling pathways represents a powerful barrier to malignant transformation and convergence on one principal driver defines a key event during leukemia-initiation. Proof-of-concept studies in patient-derived B-ALL cells revealed that small molecule agonists of STAT5 or ERK to reactivate the suppressed divergent circuits subvert oncogenic signaling and strongly synergized with direct inhibition of the principal oncogenic driver. Hence, pharmacological reactivation of divergent pathways can be leveraged as a previously unrecognized strategy to deepen treatment responses and to overcome drug-resistance. Current treatment approaches for drug-resistant cancer are focused on drug-combinations to suppress the central oncogenic driver and multiple alternative pathways. Here, we introduce a concept based on inhibition of the principal driver combined with pharmacological reactivation of divergent pathways
Conclusions: These results provide evidence that inactivation of divergent pathways that are not aligned with the principal oncogenic driver represents a critical step during malignant transformation. Unlike B-ALL, where reactivation of a divergent pathway suppresses the principal pathway and compounds toxicity, activation of an alternative pathway in solid tumors represents a route for survival and drug resistance. While current treatment approaches for drug-resistant cancer are focused on drug-combinations to inhibit multiple pathways, we introduce a scenario that is based on inhibition of the principal pathway combined with reactivation of divergent pathways.
Wiita:UCSF: Patents & Royalties; Indapta Therapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Protocol Intelligence: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Izraeli:sightdx: Consultancy; novartis: Honoraria; prime oncology: Speakers Bureau. Weinstock:Celgene: Research Funding; Verastem Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal