
Background: Current chemotherapy regimens in children with acute lymphoblastic leukemia (ALL) produce disease-free survival (DFS) rates of greater than 80%. In contrast, adults with ALL have a much poorer prognosis, with DFS rates of 40%, and less than 20% for patients over 60 years of age. The oral BCL2 inhibitor venetoclax has improved response rates in older patents with AML who are not candidates for standard induction chemotherapy. Furthermore, BH3 profiling and protein analysis has shown that ALL cell lines and primary cells exhibit BCL-2 dependence, supporting clinical trials of BCL-2 antagonists in ALL (Del Gaizo Moore et al Blood 2008; Benito et al Cell Rep 13:2715, 2015). This phase I trial was performed to determine if venetoclax can be safely added to a reduced intensity chemotherapy regimen for older adults with untreated ALL and in patients with R/R ALL.
Methods: Patients (pts) over 60 yrs or older with untreated, Philadelphia chromosome negative ALL were eligible. After the first 6 pts were enrolled, the study was amended to allow enrollment of adults 18 yrs or older with R/R ALL. The primary objective of this study was to determine the feasibility of a 21-day schedule of venetoclax with the mini-hyper-CVD chemotherapy regimen. The therapeutic backbone of this protocol was based on a lower intensity version of hyper-CVAD with no anthracycline (Kantarjian et al Lancet Oncol 2018). Two dose levels of venetoclax were tested, 400 mg and 600 mg for 21 days of each cycle. The induction chemotherapy consisted of mini-hyper fractionated cyclophosphamide, vincristine, dexamethasone, alternating with cycles of methotrexate and cytarabine. Two doses of intrathecal chemotherapy were administered per cycle during the first four cycles. Upon completion of induction/consolidation chemotherapy, patients could receive an allogeneic stem cell transplant (SCT) or receive venetoclax plus POMP maintenance chemotherapy. Bone marrow and peripheral blood samples were collected for BH3 profiling and CyTOF to measure Bcl-2 family expression. The analysis is ongoing and will be reported.
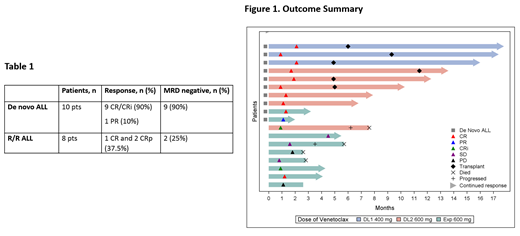
Results: To date 18 pts were enrolled. Three pts at dose level 1 (400 mg), 6 pts at dose level 2 (600 mg; the highest dose tested) and 9 pts in the expansion cohort (600 mg), which is the recommended phase II dose (RP2D). There has been no protocol defined DLTs. The median age was 65 yrs, (range, 23-82), 78% were male, 56% B-lineage, 56% were previously untreated. For the older de novo pts, 7 had B-ALL with 5 pts with haploid/near triploid cytogenetics and TP53 mutations. Two pts had ETP-ALL and 1 pt with mature T-ALL. In this group of untreated de novo pts, the overall response rate (ORR) rate was 100% with 9/10 (90%) achieving CR and 1 pt PR (Table 1 and Figure 1). All CR pts achieved an MRD negative status by multicolor flow cytometry with a sensitivity of 0.01%. Of the 10 pts with de novo ALL, 4 pts remain on treatment, and 6 pts went to SCT. The median follow up for the de novo pts was 11.3 mos (range, 2.0-17.8) and the median duration of response for these patients was 9.9 mos (range 0.9, 15.7). There have been no relapses thus far in the de novo ALL cohort. For the R/R pts, the median number of prior regimens was 2.5 (range 1-5) and the CR/CRp rate was 3/8 (37.5%) with 2 pts MRD negative (25%). Of the 8 pts with R/R ALL, 2 pts remain on treatment. For all 18 pts, the grade 3 adverse events included febrile neutropenia (39%), hyperglycemia (17%) and hypocalcemia (11%). There were two grade 4 events, pneumonia and sepsis, and no grade 5 events.
Conclusions: The administration of venetoclax with a reduced intensity chemotherapy regimen (mini-hyper-CVD) was safe and well tolerated. The RP2D dose was 600 mg daily for 21 days per cycle. This regimen was particularly well tolerated in the older previously untreated ALL population. Although the CR and MRD negative rates were exceptionally high for the older adult cohort, longer follow up and larger studies are needed. The addition of venetoclax may represent a major therapeutic advance in older adults with ALL.
Jain:Cellectis: Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Stevenson:Celgene: Research Funding. Winer:Jazz Pharmaceuticals, Pfizer: Consultancy. Garcia:Genentech: Research Funding; Abbvie: Research Funding. Stone:Arog: Consultancy, Research Funding; Otsuka: Consultancy; Biolinerx: Consultancy; Takeda: Other: DSMB; Trovagene: Consultancy; Novartis: Consultancy, Research Funding; Biosight: Consultancy; Agios: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Argenix: Other: DSMB; Amgen: Membership on an entity's Board of Directors or advisory committees; Arog: Consultancy, Research Funding; Stemline: Consultancy; Agios: Consultancy, Research Funding; Otsuka: Consultancy; Argenix: Other: DSMB; Roche: Consultancy; Macrogenics: Consultancy; Astra-Zeneca: Consultancy; Pfizer: Consultancy; Trovagene: Consultancy; Jazz: Consultancy; Biolinerx: Consultancy; Daiichi-Sankyo: Consultancy; Astellas: Consultancy; Daiichi-Sankyo: Consultancy; Celgene: Consultancy, Other: DSMB; Actinium: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Arog: Consultancy, Research Funding; Trovagene: Consultancy; Otsuka: Consultancy; Argenix: Other: DSMB; Novartis: Consultancy, Research Funding; Stemline: Consultancy; Takeda: Other: DSMB; Agios: Consultancy, Research Funding; Jazz: Consultancy; Astra-Zeneca: Consultancy; Arog: Consultancy, Research Funding; Roche: Consultancy; Macrogenics: Consultancy; Otsuka: Consultancy; Biosight: Consultancy; Abbvie: Consultancy, Research Funding; Biolinerx: Consultancy; Pfizer: Consultancy; Trovagene: Consultancy; Argenix: Other: DSMB; Biolinerx: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy; Celgene: Consultancy, Other: DSMB; Actinium: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding. Jabbour:Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Cyclacel LTD: Research Funding. Ravandi:Macrogenix: Consultancy, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Consultancy, Research Funding; Menarini Ricerche: Research Funding; Selvita: Research Funding; Cyclacel LTD: Research Funding. Kantarjian:Astex: Research Funding; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding; Cyclacel: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Research Funding; Novartis: Research Funding; Agios: Honoraria, Research Funding; Ariad: Research Funding; Amgen: Honoraria, Research Funding; Takeda: Honoraria; AbbVie: Honoraria, Research Funding; BMS: Research Funding; Pfizer: Honoraria, Research Funding. Neuberg:Pharmacyclics: Research Funding; Madrigal Pharmaceuticals: Equity Ownership; Celgene: Research Funding. Letai:AbbVie, AstraZeneca, Novartis: Consultancy, Research Funding; Zeno Pharmaceuticals, Vivid Bioscience, Flash Therapeutics, Dialectic Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Cofounder or Advisory Board member. Konopleva:Agios: Research Funding; Astra Zeneca: Research Funding; Ablynx: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Kisoji: Consultancy, Honoraria; Ascentage: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; Genentech: Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Cellectis: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Eli Lilly: Research Funding. DeAngelo:Abbvie: Research Funding; Glycomimetics: Research Funding; Amgen, Autolus, Celgene, Forty-seven, Incyte, Jazzs, Pfizer, Shire, Takeda: Consultancy; Blueprint: Consultancy, Research Funding; Novartis: Consultancy, Research Funding.
Venetoclax for patients with acute lymphoblastic leukemia.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal