Background: In adults with B-ALL, multi-agent combination chemotherapy produce high complete remission (CR) rates of 80-90%, but a significant proportion of patients relapse and the long-term cure rate is 40-50%. Monoclonal antibodies have improved survival in B-ALL both in the frontline and relapsed/refractory (R/R) setting. Blinatumomab (Blina) is a bispecific T-cell engaging (BiTE) CD19-CD3 antibody effective in the treatment of R/R B-ALL and for eradication of measurable residual disease (MRD). Better outcomes are observed when Blina is administered earlier in the course of the disease. We hypothesized that early incorporation of Blina in sequential combination with the Hyper-CVAD regimen in newly diagnosed B-ALL would improve the rates of MRD eradication, decrease the need for intensive chemotherapy, and improve survival.
Methods: This is a single-arm phase 2 clinical trial for patients (pts) ≥ 14 year-old with newly diagnosed B-ALL. Untreated pts or pts who had received ≤ 1 course of chemotherapy with PS of 0-3 and normal organ function are eligible. The treatment protocol consists of 4 alternating cycles of Hyper-CVAD and high-dose methotrexate (MTX) / cytarabine (AraC) followed by 4 consecutive cycles of Blina. Pts with CD20+ B-ALL (≥ 1% cells) receive 8 doses of rituximab (375 mg/m2) or ofatumumab (2000 mg); all pts receive 8 prophylactic intrathecal injections of MTX and AraC during the Hyper-CVAD cycles. The maintenance phase consists of 12 cycles of POMP. Blina is administered as a maintenance in all pts after every 3 cycles of POMP for 3 cycles. Allogeneic hematopoietic stem cell transplant (HSCT) is offered for high-risk pts. On June 5th 2017 after treating 10 patients, the protocol was amended: pts with high-risk features (i.e. Ph-like ALL, complex karyotype, t(4;11), low-hypodiploidy / near triploidy [Ho-Tr] or persistent MRD+) start Blina earlier after 2 cycles of Hyper-CVAD. The primary outcome is relapse-free survival (RFS) and secondary outcomes include CR rates, MRD negativity (MRD-) rates and overall survival (OS). MRD is assessed by 6-color multiparameter flow cytometry with a sensitivity of 10-4. Safety is evaluated by frequency and grading of adverse events according to the CTCAE v4.0.
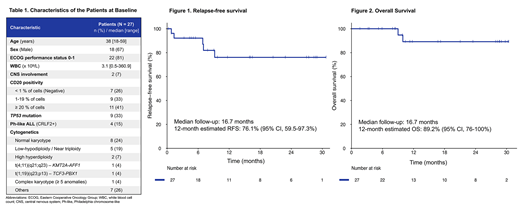
Results: As of July 3rd 2019, 27 pts were enrolled and treated, including 5 pts who were already in CR at study entry. The baseline characteristics of the pts are summarized in Table 1. The median age was 38 years (range, 18-59). Twenty (74%) pts had CD20+ expression. Fifteen (56%) pts had high-risk features at baseline: 9 (33%) with TP53 mutation, 4 (15%) with Ph-like, 5 (19%) with Ho-Tr, 1 (4%) with t(4;11) and 1 (4%) with complex karyotype.
The overall CR rate was 100% with 18/22 (82%) achieved CR after 1 cycle of Hyper-CVAD. The median time to CR was 23 days (range, 16-100). MRD negativity was achieved in 26/27 (96%) pts, of them 16/22 (73%) pts after 1 cycle of Hyper-CVAD. The only patient who did not achieve MRD- has not received Blina on protocol because of relapse after 4 cycles of Hyper-CVAD. Pts have received a median of 3 cycles (range, 2-4) of Hyper-CVAD and 2 cycles (range, 0-4) of Blina. Eight (30%) pts have undergone HSCT for high-risk features.
With a median follow-up of 17 months (range, 2-30), 4 relapses and 2 deaths were reported. One patient died from pulmonary complications after HSCT and one patient died of sepsis during re-induction chemotherapy after relapse. The 12-month estimated RFS was 76% (95% CI, 60-97%) (Figure 1) and the 12-month estimated OS was 89% (95% CI, 76-100%) (Figure 2).
The 60-day mortality rate was 0% and no death has been reported during Hyper-CVAD + Blina treatment. Grade 3-4 neurological adverse events related to Blina were reported in 4/23 (17%) pts and one grade 3 cytokine release syndrome was reported. These events were all manageable and reversible with dexamethasone and Blina interruption. Treatment has been resumed without recurrence in all but one patient with recurring grade 2 dysphasia and confusion who required discontinuation of Blina therapy. Reduction in the number of Hyper-CVAD cycles allowed to decrease the incidence of myelosuppression and febrile neutropenia.
Conclusion: The sequential combination of Hyper-CVAD and Blina in newly diagnosed B-ALL is safe and highly effective. This regimen produced CR in all pts and MRD eradication in 96% of pts. The preliminary survival outcomes are favorable. The study continues to accrue patients.
Kantarjian:Pfizer: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Novartis: Research Funding; Ariad: Research Funding; Daiichi-Sankyo: Research Funding; Takeda: Honoraria; Agios: Honoraria, Research Funding; Astex: Research Funding; Cyclacel: Research Funding; BMS: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Immunogen: Research Funding; Jazz Pharma: Research Funding; AbbVie: Honoraria, Research Funding. Short:Takeda Oncology: Consultancy, Research Funding; AstraZeneca: Consultancy; Amgen: Honoraria. Ravandi:Macrogenix: Consultancy, Research Funding; Selvita: Research Funding; Cyclacel LTD: Research Funding; Menarini Ricerche: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Consultancy, Research Funding. Garcia-Manero:Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding; Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding. Cortes:Merus: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Research Funding; Biopath Holdings: Consultancy, Honoraria; Immunogen: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Jain:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Konopleva:Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding; Forty-Seven: Consultancy, Honoraria; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Calithera: Research Funding. Takahashi:Symbio Pharmaceuticals: Consultancy. Sasaki:Otsuka: Honoraria; Pfizer: Consultancy. Wierda:Genentech: Research Funding; GSK/Novartis: Research Funding; Pharmacyclics LLC: Research Funding; Acerta Pharma Inc: Research Funding; Gilead Sciences: Research Funding; Oncternal Therapeutics Inc.: Research Funding; AbbVie: Research Funding; Miragen: Research Funding; Xencor: Research Funding; Juno Therapeutics: Research Funding; Janssen: Research Funding; Loxo Oncology Inc.: Research Funding; KITE pharma: Research Funding; Sunesis: Research Funding; Cyclcel: Research Funding. Jabbour:Adaptive: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding.
Blinatumomab is not approved for the treatment of newly diagnosed untreated acute lymphoblastic leukemia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal