Background:
The combination of low intensity therapy with InO improved survival compared to intensive chemotherapy and to single agent InO in Salvage 1 (Jabbour et al. Cancer. 2018). The sequential addition of blinatumomab (blina) may allow the administration of weekly lower dose of InO and distancing allogeneic stem cell transplant (ASCT) from the last dose of InO, while deepening the minimal residual disease response. This will lead to less veno-occlusive disease (VOD) and better long-term efficacy. The aim of this study is to evaluate the outcome of pts in first relapse treated with this combination.
Methods:
The mini-hyper-CVD (cycles 1, 3, 5, 7) comprised cyclophosphamide (150 mg/m2 every 12 h on days 1-3), vincristine (2 mg flat dose on days 1 and 8), and dexamethasone (20 mg on days 1-4 and days 11-14) without anthracycline. Even cycles (cycles 2, 4, 6, 8) comprised methotrexate (250 mg/m2 on day 1) and cytarabine (0.5 g/m2 given every 12 h on days 2 and 3). Rituximab and intrathecal chemotherapy were given for first 4 courses. InO was originally given on day 3 of the first four cycles at the dose of 1.3-1.8 mg/m2 at cycle 1, followed by 1.0-1.3 mg/m2 in subsequent cycles. After 38 pts were treated, an amendment was made to incorporate 4 cycles of blina after 4 cycles of mini-hyper-CVD + InO. InO was given on days 2 and 8 at the dose of 0.6 and 0.3 mg/m2 at cycle 1, respectively, followed by days 2 and 8 at the dose of 0.3 and 0.3 mg/m2 at subsequent cycles; blina was continuously infused over 28 days every 42-day cycle for 4 cycles. The decision to proceed with ASCT was based on the discretion of the treating physician after discussion with the pt. After amendment, all pts were placed on prophylactic ursodiol. Inverse probability of treatment weighting (IPTW) was performed after propensity score calculations using covariates at diagnosis.
Results:
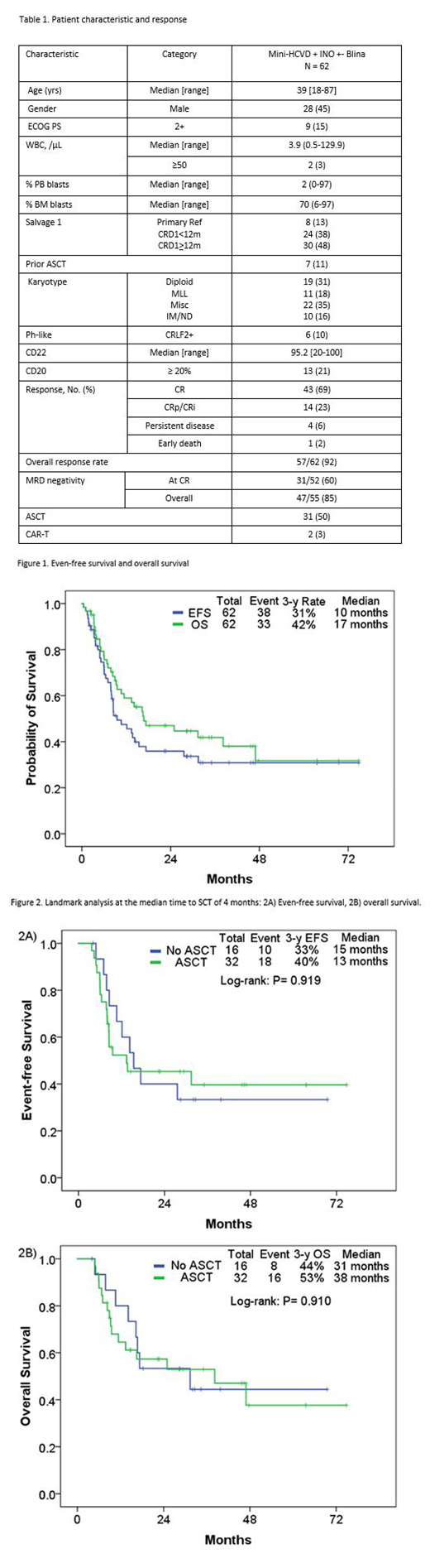
From 2/2013 to 3/2019, 62 pts were treated with mini-hyper-CVD + InO with (n=38, 55%) and without blina (n=24, 45%) as the first salvage therapy. Pt characteristics and outcome are summarized in Table 1. The median age at diagnosis was 39 years (range, 19-87). Among 62 pts, 8 (13%) pts had primary refractory disease; 24 (38%), CR1 duration less than 12 months. 7 (11%) pts had prior history of ASCT. MLL rearrangement by FISH was observed in 11 (18%) pts; CRLF2 rearrangement was observed in 6 (10%). Overall, 57 (92%) pts achieved response including CR in 43 (69%), and CRp/CRi in 14 (23%). MRD negativity by 6-color flow cytometry was achieved in 31/52 pts (60%) after 1 cycle and 47/55 pts (85%) overall. Three pts with detectable MRD before blina achieved negative MRD after blina therapy. The 30-day and 60-day mortality rates were 2% and 3%, respectively. Among 57 who achieved remission, 11 (19%) relapsed, 31 (54%) underwent allogeneic SCT in CR2, and 4 (6%) died in CR/CRp. Causes of death for pts in CR/CRp included: unknown (n=1), VOD (n=1), sepsis (n=1), and pneumonia (n=1). Overall, 6 pts (10%) developed VOD; 5 after subsequent ASCT. The rate of VOD was 5/42 (12%) in pts who did not receive blina; all 5 had ASCT-related; 1 received 2 ASCT, 1 in CR1 and 1 in CR2; 1 received ASCT in CR1; and 3 received ASCT in CR2. In contrast, only 1 case of VOD was observed among the 20 pts (5%) who received the weekly lower dose of InO and sequential addition of blina; this pt had VOD post ASCT in CR2. With a median follow-up of 34 months (range, 2-75 months), 29 pts (47%) were alive, 21 of whom (34%) were in CR and MRD negative status. The 3-year EFS and OS rates were 31% and 42%, respectively (Figure 1). The median EFS and OS were 10 and 17 months, respectively. With the landmark analysis at the median time to ASCT of 4 months (range, 1.9-9.5), the median EFS was 13 months and 15 months in pts with and without ASCT, respectively (Figure 2A; P=0.92); the median OS was 38 months and 31 months, respectively (Figure 2B; P=0.910). Using the IPTW analysis, compared to a similar historical cohort of pts treated with standard salvage chemotherapy (n=39) or Ino monotherapy (n=29), mini-hyper-CVD + InO ± blina (n=62) resulted in significantly improved survival (P<0.001; P<0.001).
Conclusion:
The combination of InO with mini-hyper-CVD +/- blina is highly effective and shows improved outcome compared to standard single agent Ino and intensive chemotherapy in pts with relapsed/refractory ALL in first relapse, with 3-year OS rate of 42%. The risk of VOD can be minimized with fractionated low dose InO and sequential combination of blina.
Sasaki:Pfizer: Consultancy; Otsuka: Honoraria. Kantarjian:Astex: Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Research Funding; Amgen: Honoraria, Research Funding; Jazz Pharma: Research Funding; Cyclacel: Research Funding; BMS: Research Funding; Immunogen: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Daiichi-Sankyo: Research Funding; Agios: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Ariad: Research Funding. Ravandi:Xencor: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Menarini Ricerche: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Selvita: Research Funding; Macrogenix: Consultancy, Research Funding. Short:Amgen: Honoraria; Takeda Oncology: Consultancy, Research Funding; AstraZeneca: Consultancy. Kebriaei:Kite: Honoraria; Amgen: Research Funding; Jazz: Consultancy; Pfizer: Honoraria. Jain:Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Incyte: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Konopleva:Agios: Research Funding; Astra Zeneca: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Ablynx: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Eli Lilly: Research Funding; Forty-Seven: Consultancy, Honoraria; Calithera: Research Funding; Amgen: Consultancy, Honoraria; Cellectis: Research Funding. Garcia-Manero:Merck: Research Funding; Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding. Champlin:Sanofi-Genzyme: Research Funding; Actinium: Consultancy; Johnson and Johnson: Consultancy. Kadia:Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Bioline RX: Research Funding; Celgene: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding. Cortes:Jazz Pharmaceuticals: Consultancy, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy; Biopath Holdings: Consultancy, Honoraria; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Research Funding. Takahashi:Symbio Pharmaceuticals: Consultancy. Jabbour:Amgen: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal