E-selectin (E-sel) is a cell adhesion glycoprotein that is expressed on endothelial cells and has been implicated in therapeutic resistance. In most myeloid leukemias, leukemic blasts express E-sel ligands (EsL), which contain the glycan epitope of the carbohydrate sialyl Lex (sLex). This expression increases the likelihood of adhesion to vascular endothelial cells and facilitates sequestration in the bone marrow vascular niche, leading to cell adhesion-mediated drug resistance and poor clinical outcome. E-sel antagonists like uproleselan, interrupts leukemic cell homing to the vascular niche, increases susceptibility to cytotoxic and targeted therapies and can be potent adjuncts to therapeutics. Recent data demonstrated a correlation between leukemic cell surface levels of EsL and response to uproleselan, linking EsL expression to response. We questioned whether transcriptome profiling of EsL-forming glycosylation genes can be used to identify elevated EsL expression in patients with acute myeloid leukemia (AML), and subsequently which patients might best respond to uproleselan.
RNA-seq data from patients treated in COG AAML1031 (N = 1,074) was available for evaluation. We examined transcriptome expression of 24 genes that code for enzymes involved in glycosylation of EsL. All analyses were performed in R. Cox proportional hazards models were generated using the survival package. Multidimensional flow cytometry (MDF) was used to detect cell surface EsL expression by two techniques: direct binding of an E-sel/hIg, PE labeled chimera, and the anti-sLex antibody HECA-452.
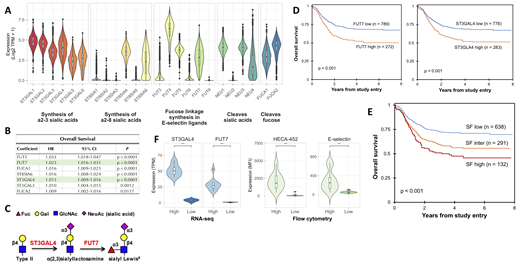
Seven of the 24 genes examined had minimal expression (mean <1 TPM) and were excluded from further analysis. The remaining 17 were variably expressed (Fig. 1A). To assess association of expression with outcome, univariate Cox models for overall survival (OS) were generated, using gene expression as a continuous coefficient (N = 1,061). Of the 17 genes, 7 were significantly associated with increased risk (p < 0.05, Fig. 1B). ST3GAL4 and FUT7 were targeted for further evaluation, as they directly synthesize sLex (Fig. 1C), and were significantly associated with adverse outcome (HR = 1.013, p < 0.0001, and HR = 1.023, p < 0.0001, respectively). Patients highly expressing FUT7 (highest quartile of expression) had significantly worse outcome than low expressors (lowest 3 quartiles of expression), with a 5-year OS of 50.3% vs. 68.3% (p < 0.0001, Fig. 1D). Similarly, those with high ST3GAL4 expression had a 5-year OS of 51.3%, compared to 68.1% for low expressors (p < 0.0001, Fig. 1D). A subset of patients highly expressed both genes (ST3GAL4 and FUT7 high; SFhigh, N = 132). Compared to patients that did not highly express either gene (SFlow), these individuals had particularly adverse survival (45.8% OS vs 71.0% OS, p < 0.0001). Patients with one of two high expressing genes (SFinter) had a 5-year OS of 55.5%, illustrating what may be a compounding unfavorable impact conferred on survival (Fig. 1E). Further investigation of clinical characteristics within these 3 groups revealed that 71.5% of infants <1 year were SFlow, with only 4.66% in SFhigh. In addition, CBF-AML was greatly underrepresented in SFhigh, with 97% of both t(8;21) and inv(16) patients in SFlow, and 0% in SFhigh.
To verify surface protein expression of the two genes, leukemic specimens from SFhigh patients (N = 10) and SFlow patients (N = 10) underwent cell surface expression evaluation of glycosylated EsL using two MDF assays. SFlow patients had low or undetectable levels of cell surface EsL by both assays, whereas SFhigh patients had significantly higher expression of EsL (p < 0.001, Fig. 1F). This suggests a strong correlation between transcriptome measurements of EsL glycosylation genes and cell surface glycosylation levels of EsL.
In summary, we have shown that multiple genes involved in the glycan synthesis of EsLs are highly expressed in pediatric AML. Two of these genes, ST3GAL4 and FUT7, are associated with poor outcome. Additionally, high expression of these genes is detectable at the transcript level and associated with cell surface EsL expression. These genes provide novel targets for overcoming drug resistance induced by the tumor microenvironment, and lend support for the use of EsL glycosylation genes as predictive biomarkers. These data also confirm the importance of E-sel in disease progression in AML and its potential as a therapeutic target.
Pardo:Hematologics, Inc: Employment. Eidenschink Brodersen:Hematologics, Inc: Employment. Magnani:GlycoMimetics Inc: Employment, Equity Ownership. Fogler:GlycoMimetics Inc: Employment, Equity Ownership. Loken:Hematologics, Inc: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal