Introduction: Inherited predisposition to myeloid malignancies in adults may be more common than previously suggested with recent studies suggesting a prevalence of candidate predisposition alleles in 15-20% of patients. An inherited predisposition may not be considered in older AML patients despite significant clinical implications for family members as potential stem cell transplant donors. To better define the role of inherited genetic alterations in older AML patients , we analyzed a unique cohort of newly diagnosed older (>60 years) patients enrolled in) Beat AML® Master Trial(BAMT) for candidate genes associated with a known or putative inherited cancer predisposition.
Methods: We analyzed extracted DNA from skin and/or saliva samples compared to paired leukemia samples of 176 AML patients enrolled in the BAMT. All samples underwent genomic profiling using a modified FoundationOne®Heme platform (capture-based) and/or the Oregon Health Sciences University panel (amplicon-based), evaluating 477 and 220 genes respectively, with a known role in hematologic malignancies. Germline(GL) variants were identified by the haplotype-based Bayesian genetic variant detector FreeBayes and using variant allele frequency(VAF) values. The pathogenicity and clinical significance of the variants was interpreted according to the 2015 ACMG/AMP guidelines while the AMP/CAP/ASCO guidelines and various disease databases were used in the somatic variant calls.
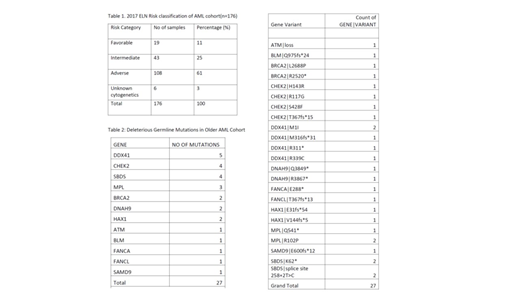
Results: -The mutational landscape of the 176 newly diagnosed older AML patients is detailed in Table 1. Our cohort has a higher proportion of adverse risk patients, consistent with an older AML patient population. 27 pathogenic or likely pathogenic GL variants were detected in 24 AML patients, with a germline mutation prevalence of 14% in this cohort. Deleterious GL mutations were found in the gene DDX41 (5), followed by SBDS (4), CHEK2 (4), MPL (3), BRCA2 (2), HAX1 (2), DNAH9 (2), FANCA (1), FANCL (1), SAMD9 (1), BLM (1), and ATM (1) (Table 2). The types of mutations included missense mutations (9), nonsense mutations (8), frameshift mutations (7), splice site mutations (2), and an exonic deletion (1). Family history of leukemia was available on 129 patients from this cohort. 12 patients have at least one family member with AML. Of these 12 patients, 2 had a deleterious GL alteration identified.
Along with the 14% prevalence of pathogenic/likely pathogenic GL mutations , there were an additional 181 GL variants of unknown significance (VUS) in 102 patients, seen in genes implicated in inherited predisposition to hematologic malignancies, most commonly variants in DOCK8 and CREBBP(>5% VUS) with both genes being implicated in leukemogenesis .
As skin and/or saliva samples were collected at the time of AML diagnosis, tumor-in-normal presence was expectedly observed. The median VAF for somatic mutations was significantly lower (p < 0.0001) in skin (median 6%; mean 9%; standard deviation (SD) 10%; N=562 variants) than in saliva (median 17%; mean 21%; SD 16%; N=368 variants). In 37 patients who had both saliva and skin tissue concomitantly ,skin had a significantly lower tumor-in-normal presence (median VAF 5%, mean 8%, SD 8%;) than saliva (median 15%, mean 20%, SD 16%)(p < 0.0001).
Conclusions: We found a prevalence of 14% pathogenic/ likely pathogenic GL mutations in cancer predisposition genes in this unique cohort of newly diagnosed older AML patients. This finding has potential clinical implications for patients and family members. We also found a large number of VUS in genes implicated in hematological malignancies. Additional studies linking candidate VUS' to familial predisposition to understand contribution to AML predisposition are needed. We are in the process of comparing the manual curation of ACMG classification of pathogenicity with a computational curation algorithm to assess the potential for automated classification of GL variants. Our study suggests the choice of source for germline DNA in patients with AML is variably impacted by leukemic contamination. Cultured skin fibroblasts are the current standard for tumor-normal paired genotyping, with the caveat of being labor intensive and not routinely performed in clinical diagnostic laboratories. This is a critical consideration for rapid GL screening of patients and family members with hematologic malignancies and suspected cancer predisposition.
Borate:Novartis: Consultancy; Takeda: Consultancy; Pfizer: Consultancy; Daiichi Sankyo: Consultancy; AbbVie: Consultancy. Mims:Abbvie: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; PTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Astellas Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Stein:Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma US, Inc: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo, Inc.: Membership on an entity's Board of Directors or advisory committees; Bioline: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; PTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees. Patel:Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dava Oncology: Honoraria; France Foundation: Honoraria. Baer:Astellas: Research Funding; Abbvie: Research Funding; AI Therapeutics: Research Funding; Forma: Research Funding; Incyte: Research Funding; Kite: Research Funding; Takeda: Research Funding. Stock:Agios: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; UpToDate: Honoraria; Daiichi: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Membership on an entity's Board of Directors or advisory committees; Research to Practice: Honoraria. Schiller:Bristol Myer Squibb: Research Funding; Celgene: Research Funding, Speakers Bureau; Constellation Pharmaceutical: Research Funding; Daiichi Sankyo: Research Funding; Agios: Research Funding, Speakers Bureau; Amgen: Other, Research Funding; Astellas: Research Funding; Biomed Valley Discoveries: Research Funding; Eli Lilly and Company: Research Funding; FujiFilm: Research Funding; Genzyme: Research Funding; Gilead: Research Funding; Incyte: Research Funding; J&J: Research Funding; Jazz Pharmaceuticals: Honoraria, Research Funding; Karyopharm: Research Funding; Novartis: Research Funding; Onconova: Research Funding; Pfizer Pharmaceuticals: Equity Ownership, Research Funding; Sangamo Therapeutics: Research Funding. Blum:AmerisourceBergen: Consultancy; Forma: Research Funding; Xencor: Research Funding; Boehringer Ingelheim: Research Funding; Celgene: Research Funding; Astellas,: Research Funding. Shami:JSK Therapeutics: Employment, Equity Ownership; Amgen: Research Funding; Pfizer: Research Funding; Cantex: Research Funding; Aptevo: Research Funding; Jazz: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Lone Star Thiotherapies: Equity Ownership. Foran:Agios: Honoraria, Research Funding. Byrd:Acerta: Research Funding; Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau; Genentech: Research Funding; Novartis: Other: Travel Expenses, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; Ohio State University: Patents & Royalties: OSU-2S; BeiGene: Research Funding; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau. Druker:OHSU (licensing fees): Patents & Royalties: #2573, Constructs and cell lines harboring various mutations in TNK2 and PTPN11, licensing fees ; Bristol-Myers Squibb: Other: PI or co-investigator on clinical trial(s) funded via contract with OHSU., Research Funding; Novartis: Other: PI or co-investigator on clinical trial(s) funded via contract with OHSU., Patents & Royalties: Patent 6958335, Treatment of Gastrointestinal Stromal Tumors, exclusively licensed to Novartis, Research Funding; Monojul: Other: former consultant; Vivid Biosciences: Membership on an entity's Board of Directors or advisory committees, Other: Stock options; Beat AML LLC: Other: Service on joint steering committee; Merck & Co: Patents & Royalties: Dana-Farber Cancer Institute license #2063, Monoclonal antiphosphotyrosine antibody 4G10, exclusive commercial license to Merck & Co; Dana-Farber Cancer Institute (antibody royalty): Patents & Royalties: #2524, antibody royalty; Aileron Therapeutics: #2573, Constructs and cell lines harboring various mutations in TNK2 and PTPN11, licensing fees , Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Patents & Royalties, Research Funding; Pfizer: Research Funding; ALLCRON: Membership on an entity's Board of Directors or advisory committees; Amgen: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Aptose Biosciences: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Beta Cat: Membership on an entity's Board of Directors or advisory committees, Other: Stock options; The RUNX1 Research Program: Membership on an entity's Board of Directors or advisory committees; Patient True Talk: Consultancy; GRAIL: Equity Ownership, Other: former member of Scientific Advisory Board; Cepheid: Consultancy, Honoraria; Blueprint Medicines: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Burroughs Wellcome Fund: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Pfizer: Other: PI or co-investigator on clinical trial(s) funded via contract with OHSU., Research Funding; ICON: Other: Scientific Founder of Molecular MD, which was acquired by ICON in Feb. 2019; CureOne: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Other: former member of Scientific Advisory Board. Vergilio:Foundation Medicine, Inc.: Employment; F. Hoffman La Roche, Ltd.: Equity Ownership. Levine:Imago Biosciences: Membership on an entity's Board of Directors or advisory committees; Prelude Therapeutics: Research Funding; C4 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Amgen: Honoraria; Gilead: Consultancy; Lilly: Honoraria; Qiagen: Membership on an entity's Board of Directors or advisory committees; Isoplexis: Membership on an entity's Board of Directors or advisory committees; Loxo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal