Introduction: Currently, there is a shortage of porcine heparin due to limited availability of porcine mucosa and supply chain issues. Bovine heparin has been used for clinical purposes globally and is being considered for reintroduction in the U.S. On a mass basis, commercially available porcine heparins exhibit a higher potency (180-220 units/mg) than their bovine counterpart (130-150 units/mg). Therefore, at gravimetric levels, the porcine heparins exhibit stronger biochemical and pharmacological effects in comparison to bovine heparin. Since heparin is standardized in biologic units relative to the USP or EP Standard, it is hypothesized that potency equated porcine and bovine heparin will exhibit similar biologic activities in laboratory assays. The purpose of this study was to compare the biologic properties of the porcine and bovine heparin at USP potency equated levels in standardized laboratory assays used for measuring and monitoring heparins.
Materials and Methods: Active pharmaceutical ingredient (API) porcine mucosal heparin (200 units/mg) of U.S. origin was obtained from Medefil Inc. (Glendale Heights, IL). Bovine heparin (140 units/mg) was obtained from KinMaster Produtos Químicos (Passo Fundo, Brazil). Whole blood Activated Clotting Time (ACT) and Thrombelastographic analyses were performed. Clot based assays such as aPTT, TT, and prothrombinase induced clotting time (PiCT) were performed using citrated human plasma supplemented with heparins up to 1 anti-Xa unit/mL. Anti-Xa and anti-IIa activities were determined using chromogenic antiprotease assays (Biophen Kits). In a purified antithrombin-supplemented system, the inhibitory effects of these agents were measured in terms of anti-Xa and anti-IIa activities and expressed as IC50 values. Thrombin generation inhibition was measured using a kinetic assay (CAT system, Diagnostica Stago, Paris, France). Protamine and platelet factor 4 neutralization profiles were investigated in plasma-based systems. The heparin induced thrombocytopenia antibody (HIT) interaction profile was assessed using platelet aggregometry.
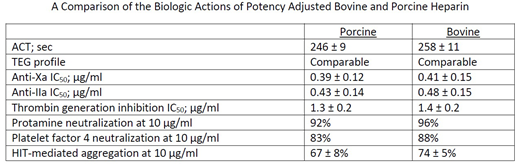
Results: Both heparins produced comparable prolongation of the ACT to a range of 200-250 seconds. The thrombelastographic profile was comparable at concentrations of 0.125 unit/mL and 2.5 units/mL. Whole blood and plasma clotting times (aPTT and TT assays) were comparable at concentrations up to 1 unit/mL. In the chromogenic anti-Xa and anti-IIa assays, the behavior of both agents was also comparable. In the purified system, the IC50 values for both agents for the anti-Xa activity ranged from 0.33-0.40 unit/mL, whereas the anti-IIa values ranged from 0.38-0.42 unit/mL; no significant differences were noted between the porcine and bovine heparins. In the thrombin generation assays, in terms of peak thrombin generation, area under the curve, and lag time, both the porcine and bovine heparins showed comparable effects. The protamine neutralization profiles of the porcine and bovine heparin exhibited variable assay dependent results. Potency adjusted bovine heparin required higher amount of protamine for the complete neutralization of the biologic effects in comparison to the porcine heparin. Similar results were obtained in the platelet factor 4 neutralization studies. In the functional HIT screening assay, both heparins exhibited a similar concentration dependent aggregation of human platelets. These results are summarized in the Table.
Summary and Conclusion: These results show that at potency adjusted concentrations, porcine and bovine heparin exhibit comparable biochemical and anticoagulant responses in whole blood, plasma based, and purified biochemical assays. Most notably, both the porcine and bovine heparin produced comparable HIT antibody mediated aggregation responses. Thus, the proposed approach to standardize heparins against a common standard using a biologic assay such as the USP method is valid. These results warrant additional validation studies to reintroduce bovine heparin for clinical use.
Jeske:KinMaster Produtos Quimicas: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal