Background:
Cerebral sinus vein thrombosis (CSVT) involves the thrombosis of the dural sinus and/or cerebral veins and it is considered a form of stroke. The estimated incidence of CSVT in children is 0.6 per 100,000 children per year. Poor outcomes, including death, happen in 9 to 29% of patients affected by CSVT. In addition, neurologic deficits, affecting primarily cognition and behavior, are seen in 50% of affected children. No randomized clinical trials have been conducted on pediatric CSVT so current guidelines for treatment have been extrapolated primarily from adult studies. Published guidelines by the American College of Chest Physicians, American Heart Association and American Society of Hematology, support the use of anticoagulation with unfractionated heparin (UFH) or low molecular weight heparin (LMWH). These same guidelines also suggest that catheter directed thrombolysis (CDT) with tissue plasminogen activator (tPA) and mechanical thrombectomy (MT) could be used when there has been clinical deterioration or no improvement (clot progression) despite anticoagulation. In all cases, these are based on uncontrolled case series and expert opinion. There is very little data on the safety and efficacy of CDT and/or MT for pediatric CSVT.
Method:
Pediatric patients with CSVT seen at Cook Children's Medical Center from January 1, 2008 to December 31, 2018 were identified by searching EMR using ICD-9 and ICD-10 codes. From this group, patients treated with MT and CDT in addition to anticoagulation were selected and reviewed.
Results:
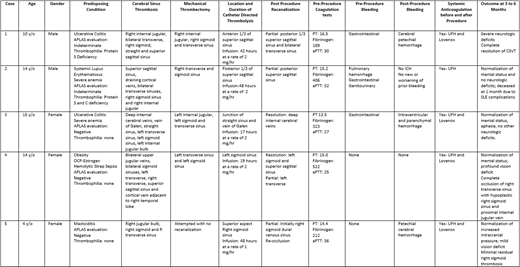
Five children (4 to 14 y/o) were treated with MT and CDT after failing anticoagulation with UFH or LMWH. Diagnosis was made by MRI/MRV and all had CSVT of multiple sinuses. Four patients had more than one underlying disorders/factors that increased their risk for thrombosis including: Ulcerative Colitis in 2, severe anemia in 2, Systemic Lupus Erythematosus (SLE) in 1, use of oral contraceptives together with obesity and bacterial sepsis in 1. Two patients did have a thrombophilia: Protein S deficiency in 1 and Protein S and C deficiency in another. One patient with SLE had a positive hexagonal phase neutralization test but rest of evaluation was negative. Three patients had systemic bleeding prior initiation of UFH and MT/CDT. All children were treated with UFH, and due to clinical neurologic deterioration and/or worsening of imaging findings (4 comatose and 1 with persistent increased ICP), all underwent thromboaspiration and catheter directed infusion of tPA for 17 to 48 hours at a dose of 1 to 2 mg/hr. All patients continued anticoagulation with UFH during catheter directed tPA infusion and after the catheter was removed. All cases had partial resolution of the sinus vein thrombosis, although 1 had quick reocclusion. Post procedure bleeding happened in 1 patient who had also had an external ventricular drainage placed and developed parenchymal and intraventricular hemorrhage that led to discontinuation of tPA infusion, and 2 patients developed petechial brain hemorrhages. Four patients had great neurologic recovery and minimal deficits, but 1 had significant neurologic deficits. One patient died from lupus complications. (Table)
Conclusion:
Endovascular therapy including MT and CDT with tPA in conjunction with systemic UFH, may have a role in pediatric patients with CSVT who have deterioration despite initial anticoagulation. In our series, after procedures, all patients had partial resolution of their CSVT (but 1 had quick reocclusion) and 4 out of 5 patients had good neurologic outcomes despite bad predictor signs (coma, extensive CSVT). Further studies are needed to identify which patients would benefit from early endovascular treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal