Background: Reactive oxygen species (ROS) play an important role in the complex and multifactorial pathophysiology of hereditary hemolytic anemia like sickle cell disease (SCD), β-thalassemia and hereditary xerocytosis (HX). Increased intracellular levels of oxidative stress disrupt normal cell functioning and may contribute to premature red blood cell (RBC) clearance from the circulation. Pyruvate kinase (PK) is a key regulatory enzyme of glycolysis, the cell's main source of energy. Because PK is very sensitive to redox balance we hypothesized that increased levels of oxidative stress in SCD, β-thalassemia and HX impairs proper enzyme function, thereby compromizing RBC energy metabolism. This may contribute to disease pathophysiology.
Aims: To investigate if secondary deficiency of PK is common in SCD, thalassemia, and HX, and to investigate if PK in these disorders is able to respond to treatment with the allosteric PK activator AG-348 (mitapivat).
Methods: Enzymatic activities of red cell PK and hexokinase (HK) were measured together with PK-thermostability in order to assess relative PK activity and enzyme stability. Purified RBCs were incubated with AG-348 (3.33μM) for 24 hours after which PK activity and ATP response was measured. RBCs of SCD patients were also analyzed with the oxygenscan, a newly developed method that characterizes individual sickling behavior by oxygen gradient ektacytometry (Rab et al, Am J Hematol, 2019). Individual tendency to sickle is reflected by Point-of-Sickling (PoS) that indicates the specific pO2 at which RBCs start to sickle during deoxygenation under shear stress.
Results: Thirty-eight patients and 21 healthy controls (HC) were included. The patient cohort consisted of patients homozygous for HbS (HbSS, n=26), patients compound heterozygous for HbS and HbC (HbSC, n=4), β-thalassemia major (regularly transfused, n=3), and hereditary xerocytosis (n=5).
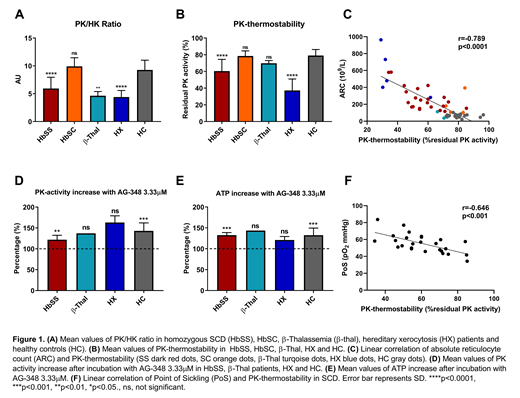
Patients showed reticulocytosis and, in line with this, a concomitant increase in HK activity. In contrast however, relative PK activity was decreased significantly compared to HK in HbSS, β-thalassemia and HX patients, but not in HbSC patients (Figure 1A). PK thermostability was significantly decreased compared to healthy controls in HbSS patients and patients with HX (Figure 1B). In HbSC and β-thalassemia patients, PK-thermostability was comparable to HC. PK thermostability strongly correlated with absolute reticulocyte count (ARC), indicating that patients displaying the highest degree of PK instability had the highest reticulocyte count (Figure 1C). This suggests that in general, a higher degree of PK instability is associated with more severe anemia due to a high hemolytic rate. In SCD patients, PK-thermostability inversely correlated with PoS, indicating that decreased PK stability is associated with sickling at higher pO2 (r=-0.646, p<0.001, Figure 1F).
When purified RBCs were incubated with 3.33μM of the allosteric PK-activator AG-348, an increase in PK activity was seen in all patients and HCs, with a mean increase of 122% in HbSS (range 111-139%, n=6), 137% in β-thalassemia (n=1), 163% in HX (range 152-174%, n=2) and 143% in HC (range 113-173%, n=9, Figure 1E). Accordingly, ATP-levels increased in all patients and HCs, with a mean increase of 133% in HbSS (range 125-141%, n=5), 144% patient with β-thalassemia (n=1), 121% in HX (range 112-129, n=3), and 132% in HCs (range 101-149%, n=9, Figure 1E).
Conclusion: PK enzyme activity and stability is compromised in patients with various forms of hereditary hemolytic anemia. This implies that PK stability and, hence, compromised red cell metabolism could contribute to the complex pathophysiology of these diseases. In SCD patients, reduced PK-thermostability is associated with higher PoS, which we previously have shown to be associated with more severe disease (Rab et al, Am J Hematol, 2019, ASH 2019 abstract ID128870). This is confirmed by the correlation of decreased PK-thermostability with increased reticulocyte count as presented in this study.
Current studies are in progress to further substantiate the underlying mechanism(s) involved, and to investigate whether AG-348 may ameliorate clinical features such as hemolysis, sickling tendency and iron overload.
Rab:RR Mechatronics: Research Funding. Bos:RR Mechatronics: Research Funding. Kosinski:Agios Pharmaceuticals, Inc: Employment, Other: Stakeholder. Kung:Agios Pharmaceuticals, Inc: Employment, Other: Stakeholder. van Beers:Agios Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Research Funding; RR Mechatronics: Research Funding. van Wijk:Agios Pharmaceuticals: Consultancy, Research Funding; RR Mechatronics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal