Background: Long-term survivors of Hodgkin lymphoma (HL) experience neurocognitive impairment despite receiving no CNS-directed therapy, though little is known about the biologic mechanisms underlying this impairment. This study aimed to examine associations between pro-inflammatory and oxidative stress biomarkers with neurocognitive impairment in long-term HL survivors.
Methods: 197 HL survivors (mean[SD] current age 36[8] years, 22[8] years post-diagnosis) and 199 age-, sex- and race/ethnicity-frequency-matched community controls completed neurocognitive testing (intelligence, attention, processing speed, memory) and systemic biomarker assessment. Neurocognitive scores were converted into age-adjusted Z-scores (μ=0, σ=1.0) using national normative data. Pro-inflammatory cytokines (IL-6, TNF-α) and markers of oxidative stress (malondialdehyde [MDA], oxidized low density lipoprotein, glutathione peroxidase [GPx]) were measured using multiplex assays on a Luminex platform. Biomarkers with undetectable concentrations were set at the lower limits of detection. Tertiles of each biomarker were created based on distributions in controls. Generalized linear models (GLM) compared survivors and controls on neurocognitive Z-scores adjusting for sex and on biomarker concentrations adjusting for sex and age. Sex-adjusted GLM compared the neurocognitive Z-scores across tertiles of each biomarker separately in HL survivors and controls. The analysis was limited to neurocognitive tests where survivors performed worse than controls after adjustment for the false discovery rate.
Results: HL survivors demonstrated worse verbal reasoning (mean Z-score -0.3 vs. 0.1, p<0.001), focused (0.3 vs.0.6 p=0.034) and sustained attention (-0.1 vs. 0.2 p=0.005), short-term (-0.2 vs. 0.1 p=0.003) and long-term memory (-0.3 vs. 0.04 p=0.005), and fine motor (-0.4 vs 0.0 p<0.001), visual (0.3 vs 0.6 p<0.001), and visuomotor processing speed (0.1 vs 0.4 p=0.004) compared to controls.
HL survivors, when compared to controls, demonstrated higher concentrations of TNF-α (β=0.9 95%CI 0.2, 1.6, p=0.012), IL-6 (β=0.8 95%CI 0.2, 1.5, p=0.013), oxidized low density lipoprotein (β=22.1 95%CI 14.1, 30.0, p<0.001), and GPx (β=54.5 95%CI 27.8, 81.2, p<0.001).
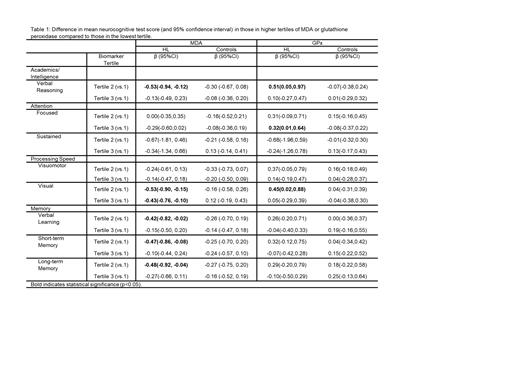
Mean difference in neurocognitive tests scores across tertiles of MDA and GPx are presented in Table 1. Among HL survivors, higher concentrations of MDA were associated with worse verbal reasoning (β =-0.5 95%CI -0.9, -0.1), visual processing speed (β =-0.4 95%CI -0.8, -0.1), verbal learning (β =-0.4, 95%CI -0.8, -0.02), and short- (β =-0.5 95%CI -0.9, -0.1) and long-term memory (β =-0.5 95%CI -0.9, -0.04). Higher concentrations of GPx were associated with better verbal reasoning (β =0.51 95%CI 0.05, 0.97), visual processing speed (β =0.45 95%CI 0.02, 0.88) and focused attention (β =0.32 95%CI 0.01, 0.64) in HL survivors. No associations between neurocognitive function and MDA or GPx were found among community controls, suggesting HL survivors may be particularly susceptible to oxidative stress. No statistically significant associations with oxidized LDL were noted among survivors or controls.
Among HL survivors, higher concentrations of IL-6 were associated with worse sustained attention (β=-1.1 95%CI -2.2, -0.02), and visuomotor (β=-0.4 95%CI -0.7, -0.01) and visual processing speed (β=-0.4 95% CI -0.7, -0.01). Among controls, these associations were less pronounced (β =-0.39 95%CI -0.69, -0.10, β =-0.41 95%CI -0.74, -0.09, and β =-0.10 95%CI -0.45, 0.24 respectively). Higher concentrations of IL-6 were associated with worse verbal learning and long-term memory in controls but not in HL survivors, suggesting the mechanisms by which IL-6 is associated with cognitive impairment may differ in survivors. Higher concentrations of TNF-α were associated with better long-term memory in survivors (β=0.6 95% CI 0.1, 1.0) but not controls.
Conclusion: HL survivors have persistent neurocognitive impairment, inflammation, and oxidative stress up to 20 years after treatment. Associations between oxidative stress and intelligence, processing speed and memory deficits were unique to HL survivors, identifying potential causal pathways that warrant further research to understand the pathophysiology of neurocognitive impairment in HL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal