Background: Ibrutinib (IBR), a BTK inhibitor, and venetoclax (VEN), a BCL-2 inhibitor are approved for patients (pts) with CLL. We recently reported results of the first-line cohort (n=80) of an investigator-initiated phase II trial of combined IBR and VEN for pts with CLL (Jain N et al. NEJM 2019) (NCT02756897). Here we report updated data for these 80 pts with focus on MRD results.
Methods: Pts with previously untreated CLL meeting 2008 IWCLL treatment criteria were enrolled. All pts had at least one of the following features: del(17p), mutated TP53, del(11q), unmutated IGHV, or an age of 65 years or older. Pts received IBR monotherapy (420 mg daily) for 3 cycles followed by addition of VEN (weekly dose-escalation to 400mg daily target dose). Combined therapy was administered for 24 cycles. Pts with bone marrow (BM) undetectable MRD (U-MRD) (assessed by multi-color flow cytometry; sensitivity 10-4) at 24 cycles of combined therapy will stop both VEN and IBR; MRD+ pts could continue IBR. Response assessments were performed using blood, BM and CT imaging (2008 IWCLL criteria) at the following time-points (after cycle 3 of IBR monotherapy, and then after cycles 3, 6, 9, 12, 18, and 24 of the combination therapy). Progression-free survival (PFS) was assessed as the time from the start of study drug to CLL progression, Richter transformation, or death. Overall survival (OS) was assessed as the time from the start of study drug to death.
Results: A total of 80 pts were enrolled. The median age was 65 years (26-83). The baseline characteristics are shown in Table 1. A total of 30% of the pts were 70 years of age or older. Overall, 92% of the pts had unmutated IGHV, TP53 aberration, or chromosome 11q deletion. The median follow-up for all pts is 22.8 months.
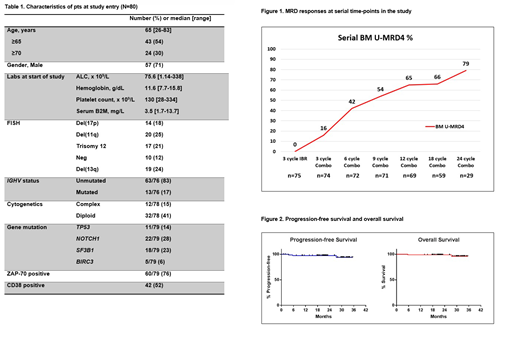
Five pts came off study during IBR monotherapy (reasons listed below). 75 pts initiated VEN. Serial BM MRD responses are shown in Figure 1. After 3 cycles of IBR monotherapy, none of the 75 pts had achieved BM U-MRD. After addition of VEN, increasing proportions of pts achieved BM U-MRD remission. After 3 cycles of the combination, 12/74 (16%) achieved BM U-MRD remission. After 6 cycles of the combination, 30/72 (42%) achieved BM U-MRD remission. After 12 cycles of the combination, 45/69 (65%) achieved BM U-MRD remission. After 24 cycles of the combination, 23/29 (79%) achieved BM U-MRD remission.
PFS and OS are shown in Figure 2. No pt has had CLL progression. Richter's transformation developed in one pt; this was a 63-year-old man with CLL with high-risk genomics (unmutated IGHV and mutated NOTCH1) in whom back pain developed during dose escalation of venetoclax and who was noted to have DLBCL transformation. Two pts died. One pt was a 60-year-old man who was having headache and numbness on the right side for 1 week before starting ibrutinib. The pt received 1 day of ibrutinib monotherapy, had progressive neurologic symptoms, and was found to have CNS cryptococcal infection. Ibrutinib was discontinued and the pt died 6 months later from complications of disseminated cryptococcal infection. This was deemed unrelated to ibrutinib as the pt had symptoms prior to starting ibrutinib. A second pt received only 2 weeks of ibrutinib and was taken off trial due to development of fungal pneumonia. The pt continued ibrutinib (off trial) and died 2 years later from infectious complications.
A total of 12 (15%) pts have come off trial. Five pts came off trial during IBR monotherapy [skin rash, n=1; hypertension, n=1; prohibited medication, n=1; unrelated infection (cryptococcus), n=1; withdrew consent, n=1]. Seven pts came off study during the combination phase [recurrent neutropenia, n=2; DLBCL transformation, n=1; pneumonia, n=1; fallopian tube cancer, n=1; allogeneic SCT, n=1; hemolytic anemia/MDS, n=1]. 54% pts had dose reduction of IBR; 29% had dose reduction of VEN.
Conclusions: Combined IBR and VEN is an effective chemotherapy-free oral regimen for pts with high-risk previously untreated CLL. Ongoing randomized studies will further help define the role of this combination approach in CLL.
Jain:BMS: Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding. Thompson:AbbVie: Research Funding; Gilead: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Pharmacyclics: Research Funding; Pfizer: Research Funding; Amgen: Consultancy, Research Funding. Burger:BeiGene: Research Funding; Pharmacyclics, an AbbVie company: Research Funding; Aptose Biosciences, Inc: Research Funding; AstraZeneca: Honoraria; Gilead Sciences: Research Funding; Janssen Pharmaceuticals: Consultancy, Honoraria. Borthakur:Cyclacel: Research Funding; GSK: Research Funding; Janssen: Research Funding; Incyte: Research Funding; AbbVie: Research Funding; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Research Funding; Bayer Healthcare AG: Research Funding; BioTheryX: Membership on an entity's Board of Directors or advisory committees; Argenx: Membership on an entity's Board of Directors or advisory committees; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Cantargia AB: Research Funding; Merck: Research Funding; Arvinas: Research Funding; Polaris: Research Funding; Strategia Therapeutics: Research Funding; Tetralogic Pharmaceuticals: Research Funding; Eisai: Research Funding; Xbiotech USA: Research Funding; Novartis: Research Funding; NKarta: Consultancy; Oncoceutics, Inc.: Research Funding; BMS: Research Funding; Oncoceutics: Research Funding; Agensys: Research Funding; PTC Therapeutics: Consultancy; Eli Lilly and Co.: Research Funding. Takahashi:Symbio Pharmaceuticals: Consultancy. Fowler:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kadia:Bioline RX: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding. Konopleva:Agios: Research Funding; Astra Zeneca: Research Funding; Calithera: Research Funding; Ablynx: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Kisoji: Consultancy, Honoraria; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Ascentage: Research Funding; Genentech: Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Cellectis: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Eli Lilly: Research Funding. Alvarado:Abbott: Honoraria; Jazz Pharmaceuticals: Research Funding. DiNardo:jazz: Honoraria; celgene: Consultancy, Honoraria; agios: Consultancy, Honoraria; syros: Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; abbvie: Consultancy, Honoraria; daiichi sankyo: Honoraria; medimmune: Honoraria. Bose:Incyte Corporation: Consultancy, Research Funding, Speakers Bureau; Celgene Corporation: Consultancy, Research Funding; Blueprint Medicine Corporation: Consultancy, Research Funding; Kartos: Consultancy, Research Funding; Constellation: Research Funding; Pfizer: Research Funding; Astellas: Research Funding; NS Pharma: Research Funding; Promedior: Research Funding; CTI BioPharma: Research Funding. Pemmaraju:mustangbio: Consultancy, Research Funding; abbvie: Consultancy, Honoraria, Research Funding; samus: Research Funding; celgene: Consultancy, Honoraria; cellectis: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; novartis: Consultancy, Research Funding; plexxikon: Research Funding; Daiichi-Sankyo: Research Funding; sagerstrong: Research Funding; affymetrix: Research Funding; incyte: Consultancy, Research Funding. Jabbour:Pfizer: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Takeda: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Amgen: Consultancy, Research Funding. Sasaki:Otsuka: Honoraria; Pfizer: Consultancy. Garg:Garglet LLC: Other: Owner; Enlitic inc.: Other: Advisor. Plunkett:Cyclacel Ltd: Research Funding. Kantarjian:Agios: Honoraria, Research Funding; BMS: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Research Funding; Novartis: Research Funding; Cyclacel: Research Funding; Ariad: Research Funding; Takeda: Honoraria; Pfizer: Honoraria, Research Funding; Astex: Research Funding; Immunogen: Research Funding; Daiichi-Sankyo: Research Funding; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding. Wierda:Janssen: Research Funding; Xencor: Research Funding; Loxo Oncology Inc.: Research Funding; Cyclcel: Research Funding; Oncternal Therapeutics Inc.: Research Funding; Miragen: Research Funding; GSK/Novartis: Research Funding; Sunesis: Research Funding; AbbVie: Research Funding; Genentech: Research Funding; KITE pharma: Research Funding; Juno Therapeutics: Research Funding; Pharmacyclics LLC: Research Funding; Gilead Sciences: Research Funding; Acerta Pharma Inc: Research Funding.
Combination of ibrutinib and venetoclax is not FDA approved
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal