Introduction:
Identification of the right drug, for the right patient, at the right time is the defining goal for precision and personalized medicine programs. Many tools are currently being pursued for this including next-gen sequencing and other -omics approaches to determine target availability (Moscow et al, 2018 Nat Rev Clin Oncol.), functional drug response profiling in viable patient samples (Snijder et al, 2017 Lancet Haem), or a combination of both (Schmidl et al, 2019 Nat Chem Bio). Of those, functional precision medicine approaches are only just beginning to gain clinical interest, and because of this, nearly all programs lack systematic testing in prospective clinical studies (Letai, 2017 Nat Rev Med).
One of the first functional precision medicine approaches to be tested in a small prospective clinical study was the use of high-throughput image-based screening and single cell analysis of drug response in primary tissues ("Pharmacoscopy") as a tool to prospectively rank drug options for patients with late-stage hematological indications (Snijder et al 2017, Lancet Haem, NCT03096821). In this study, taking Pharmacoscopy data into account for treatment selection led to a higher overall response rate (88% vs. 24%) and longer progression free survival (22.6 vs. 5.7 weeks) compared to the previous line of therapy.
In order for functional precision medicine programs to reach widespread routine use, ultimately the development of fully-certified in vitro diagnostic test products will be required. This requires amongst others a systematic understanding of i) the technical robustness of functional drug testing approaches and ii) a detailed understanding of how pre-analytical sample handling influences assay results.
Here, we set out to investigate two crucial questions that arose in the course of systematic assay development for AML: a) do CD34+ cells derived from peripheral blood and bone marrow AML patients respond differently to short term ex vivo treatment with small molecule drugs and b) does the overall response of these cells change upon cryopreservation? These issues are of high relevance even outside the setting of functional precision medicine development programs given worldwide biobanking efforts, and the lack of robust systematic model comparison.
Methods:
Fresh patient blood and bone marrow samples of newly diagnosed as well as relapsed and refractory patients with AML were collected at Medical University of Graz under appropriate ethics approval. Samples were divided into two parts and one part used immediately, the other cryopreserved as viable cells. The response of CD34+ cells against 140 different small molecule drugs (two concentrations and three technical replicates) was evaluated using single-cell high content microscopy (Allcyte's "Pharmacoscopy" platform) in both peripheral blood and bone marrow as well as freshly used and previously viably frozen samples. Drug response was determined by fitting to generalised linear and mixed models. Further, RNA is isolated from additional patient samples before and after biobanking for Nanostring analysis.
Results:
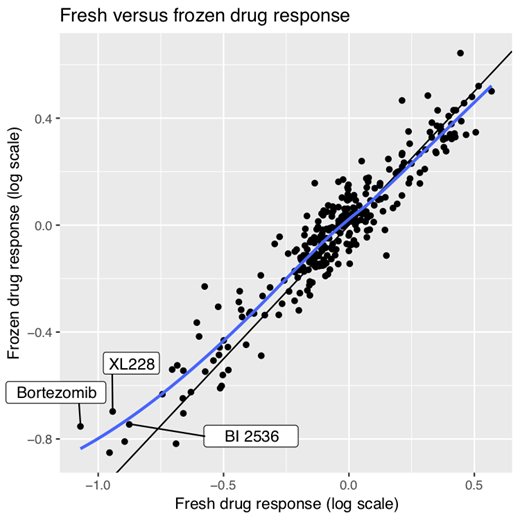
Overall, across all tested patient samples, we observed a high correlation between drug response tested in blood and bone marrow samples from this group of patient samples, indicating, at least for AML, no strong niche dependence for the drugs tested. Additionally, we also observed a high correlation between drug response tested in fresh and frozen tissues (Figure 1). The differences that did appear were, in particular, in drugs targeting metabolic and cell-growth dependent functions, amongst others.
Conclusions:
Understanding how biobanking affects the ex vivo drug response of primary human tissues is paramount to being able to understand how far translational efforts can go using tissues and functional drug response assays. We provide systematic data supporting the use of cryopreserved samples for the functional analysis of drug response in AML. This is a fundamental question associated with the use of biobanks for investigations of primary patient material.
Krall:Allcyte: Employment, Equity Ownership. Meszaros:Allcyte: Employment. Vilagos:Allcyte: Employment. Sill:Astellas: Other: Advisory board; Novartis: Other: Advisory board; Astex: Other: Advisory board; AbbVie: Other: Advisory board. Vladimer:Allcyte: Employment, Equity Ownership, Patents & Royalties: EP3198276A1.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal