Nucleic acid medicines including small interfering RNAs (siRNAs) are expected to be next-generation therapies. However, targeted delivery of siRNAs into cancer cells, especially hematological cancer cells, via systemic administration is limited by the lack of efficient and cell-specific delivery systems. We previously demonstrated that cancer cell-derived exosomes are preferentially transfer into their parental cancer cells. Based on this observation, we hypothesized that monoclonal antibody (mAb)-conjugated siRNAs that capture exosomes can efficiently deliver siRNAs into hematological cancer cells (Figure 1). To test this hypothesis, we developed a noncovalent Ab-conjugated siRNA system and confirmed the efficient delivery into multiple myeloma (MM) cells. We selected the surface antigen CD63 to capture MM cell-derived exosomes. An anti-CD63 mAb was thiolated using Traut's reagent and then modified with a linear (D-arginine (Arg))9 (9r) or branched (Arg)18 (9+9r) peptide (vehicle). The anti-CD63 mAb-Arg:siRNA complex was obtained by mixing anti-CD63 mAb-Arg and siRNA in phosphate-buffered saline and incubating the mixture.
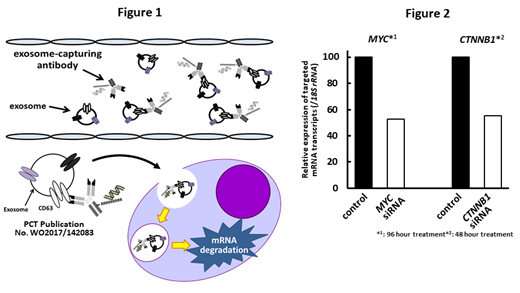
A gel shift assay showed that the anti-CD63 mAb-linear Arg construct bound to siRNAs in a construct concentration-dependent manner and the optimal vehicle-to-siRNA ratio was 5:1. By contrast, the anti-CD63 mAb-branched Arg construct bound to siRNAs at lower concentrations, and the optimal vehicle-to-siRNA ratio was 1:1-2.5:1. We next examined uptake of fluorescence-labeled siRNAs by OPM-2 MM cells using a confocal microscope. A higher level of siRNAs was delivered using the anti-CD63 mAb-branched Arg construct than using the anti-CD63 mAb-linear Arg construct; therefore, we used the former construct for further experiments. We investigated the knockdown effects of the CD63 mAb-branched Arg construct conjugated with luc siRNA in luciferase-expressing OPM-2 cells (OPM-2-luc cells). Luciferase activity decreased to 55.9% in OPM-2-luc cells following uptake of this construct. We next examined the mRNA transcript levels of MYC and CTNNB1 (β-catenin) mRNA transcript levels by RT-PCR in OPM-2 cells treated with the anti-CD63 mAb-branched Arg construct conjugated with MYC and CTNNB1 siRNAs. Treatment with mAb-conjugated siRNAs at a vehicle-to-siRNA ratio of 1:1 decreased the mRNA transcript levels of MYC and CTNNB1 to 52.5% and 55.3%, respectively (Figure 2).
In conclusion, exosome-capturing antibody-conjugated siRNAs are effectively delivered into MM cells via uptake of exosomes by parental cells. We are currently investigating their delivery into other hematological cancer cells. This technology might lead to a breakthrough in drug delivery systems for hematological cancers.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal