Background: Multiple myeloma (MM) is a heterogeneous disease with varying survival outcomes depending on the presence of certain genetic abnormalities. Common abnormalities include trisomies, translocations involving the chromosome 14, and amplifications or deletions of chromosomes 1, 13, and 17. t(11;14), occurring in 15% of patients with myeloma, had been considered a standard risk abnormality, but recent data suggest inferior outcome. This is important as new therapeutic options such as the BCL-2 inhibitor venetoclax has been shown to be particularly effective in t(11;14) patients.
Methods: This was a multicenter study to identify the outcomes of patients with t(11;14), using a retrospectively assembled cohort. Patients with MM diagnosed between 2005 and 2015 with t(11;14) identified on FISH performed within six months of diagnosis, and with treatment details available and if alive, a minimum of 12 months of follow up, were enrolled.
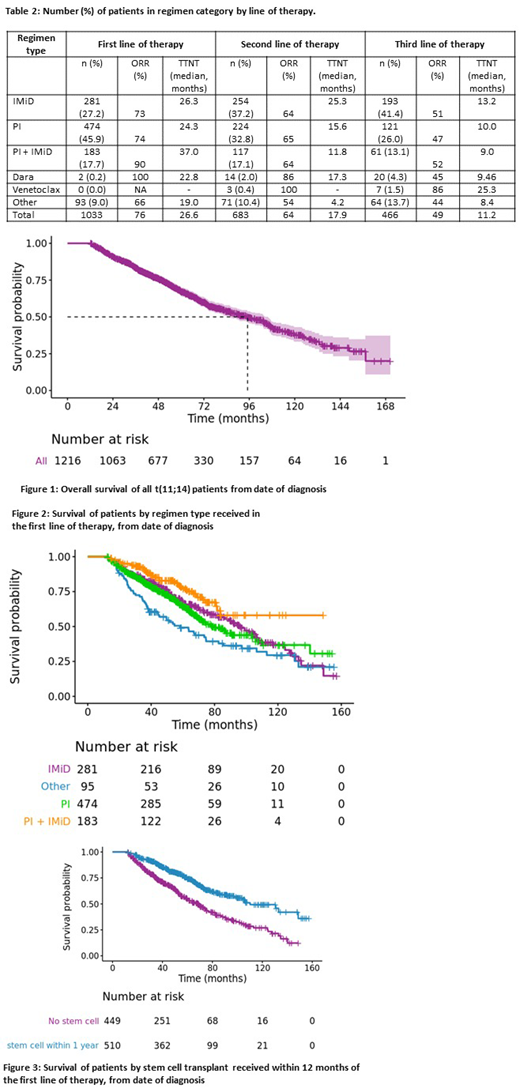
Results: The current analysis includes 1216 patients; median age of 62.56 years; 58.7% male. The median follow-up from diagnosis for the entire cohort was 51.9 months; 69.1% of the patients were alive at the last follow up. ISS stage distribution included: Stage I (35.7%), Stage II (34.0%) and Stage III (15.1%), data was missing for the rest. The distribution of concurrent FISH abnormalities included: trisomies (3.5%), del 13q (13.3%), 1q amp (8.8%), and del 17p or monosomy 17 (5.8%). Initial regimen included: 27.2% had an immunomodulatory (IMiD), 45.9% had a proteasome inhibitor (PI), 17.7% had both, and 9.0% had no novel agent. The drug classes by line of therapy are shown in Table 1. An early stem cell transplant (defined as within 12 months of start of first line treatment) was used in 49.4% of patients. The median time to next treatment (TTNT) after starting initial treatment was 26.6 (95% CI: 23.9 to 29.2) months. The median overall survival (OS) from diagnosis for the entire cohort was 95.1 (95% CI: 85.9 to 105.9) months; 4-year estimates for those diagnosed from January 2005 to December 2009, and from January 2010 to December 2014 were 77.5% and 78.6%, respectively. The median OS for those with any one high risk FISH lesion (del 17p/ 1q amp) was 67.5 (55.2, 97.1) versus 101.7 (89.7, 107.3) months. Patients with early SCT (within 12 months of diagnosis) had better OS: 108.3 (103.8, 133.0) vs. 69.8 (61.5, 80.3) months.
Conclusion: Patients with t(11;14) without high risk FISH abnormalities have an excellent survival. Patients receiving a PI + IMiD combination and those receiving autologous SCT as part of initial therapy had best survival. Though numbers are limited, patients in the later lines receiving newer drugs such as venetoclax and daratumumab had high response rates and durable responses.
Kumar:Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Takeda: Research Funding. Bittrich:Celgene: Other: Travel Funding, Research Funding; Else Kröner Fresenius Foundation: Research Funding; Otsuka Pharmaceuticals Europe: Other: N/A; SANOFI Aventis: Membership on an entity's Board of Directors or advisory committees, N/A, Research Funding; University Hospital Wuerzburg: Employment; Bristol Myers Squibb: Research Funding; Pfizer: Other: Travel Funding; AMGEN: Other: Travel Funding; JAZZ Pharmaceuticals: Other: Travel Funding; Wilhelm Sander Foundation: Research Funding; German Research Foundation (DFG): Other: N/A; University of Würzburg: Other: N/A. Goldschmidt:Mundipharma: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; John-Hopkins University: Research Funding; Dietmar-Hopp-Stiftung: Research Funding; Janssen: Consultancy, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Research Funding; Molecular Partners: Research Funding; John-Hopkins University: Research Funding; Amgen: Consultancy, Research Funding; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Chugai: Honoraria, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Reece:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Otsuka: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Merck: Research Funding. Mateos:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmamar: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Adaptive: Honoraria; EDO: Membership on an entity's Board of Directors or advisory committees. Ludwig:Celgene: Speakers Bureau; Amgen: Research Funding, Speakers Bureau; Takeda: Research Funding, Speakers Bureau; PharmaMar: Consultancy; Janssen: Speakers Bureau; BMS: Speakers Bureau. Mangiacavalli:celgene: Consultancy; Amgen: Consultancy; Janssen cilag: Consultancy. Dimopoulos:Sanofi Oncology: Research Funding. Kastritis:Amgen: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Takeda: Honoraria; Pfizer: Honoraria; Prothena: Honoraria; Genesis: Honoraria. Yee:Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Karyopharm: Consultancy; Adaptive: Consultancy. Raje:Amgen Inc.: Consultancy; Bristol-Myers Squibb: Consultancy; Celgene Corporation: Consultancy; Takeda: Consultancy; Janssen: Consultancy; Merck: Consultancy. Rosta:Cornerstone Research Group: Employment. Haltner:Cornerstone Research Group: Employment. Cameron:Cornerstone Research Group: Employment, Equity Ownership. Durie:Amgen, Celgene, Johnson & Johnson, and Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal