Background:
The 2008 International Working Group for CLL (IWCLL) criteria define MBL as a clonal B-cell disorder with a peripheral blood B-cell count of <5 x 109/L, in the absence of cytopenias(s), lymphadenopathy and/or organomegaly on physical examination, and no other concomitant lymphoproliferative disorder. In the presence of palpable lymphadenopathy and/or organomegaly, and a B-cell count of < 5 x 109/L, a diagnosis of small lymphocytic lymphoma (SLL) is established. A study by Gentile et al (Am J Hematol. 2013) reported that 29 out of 62 individuals with clinically identified MBL (42%) had lymphadenopathy identified on CT imaging. Notably, however, after a median follow-up of 35 months, the rate of progression among cases of MBL with lymphadenopathy identified on CT imaging was only 6.9% (2/29 patients), which was no different than in cases of MBL without lymphadenopathy on CT imaging (rate of progression, 9%; 3/33 patients). Our study sought to 1) determine if lymphadenopathy and/or organomegaly detected by imaging studies performed for unrelated reasons in individuals with MBL can identify a subset with shorter time to first CLL therapy (TFT) and overall survival (OS); and 2) compare outcomes of individuals with MBL who have incidentally detected lymphadenopathy/splenomegaly and SLL patients.
Methods:
We used the Mayo Clinic CLL Database to identify individuals with high-count MBL (absolute B-cell count: 0.5 - 4.9 x 109/L) and divided them into the following three cohorts: a) Cohort A: no imaging studies available at MBL diagnosis; b) Cohort B: imaging studies done within one year of MBL with no evidence of lymphadenopathy and/or organomegaly; and c) Cohort C: imaging studies done within one year of MBL with lymphadenopathy and/or organomegaly, attributable to MBL. All individuals with MBL were first seen at Mayo Clinic after 1/1/2002; those with a concomitant lymphoproliferative disorder within 3 months of diagnosis were excluded. Patients with SLL seen during the same time interval were identified as a comparison cohort. We compared the baseline characteristics (Kruskal Wallis for continuous and Chi-square for categorical variables) and TFT and OS across the three MBL cohorts, and patients with SLL. Cumulative incidence of TFT was adjusted for the competing risk of death. Age- and sex-adjusted OS was analyzed using inverse weights methods.
Results:
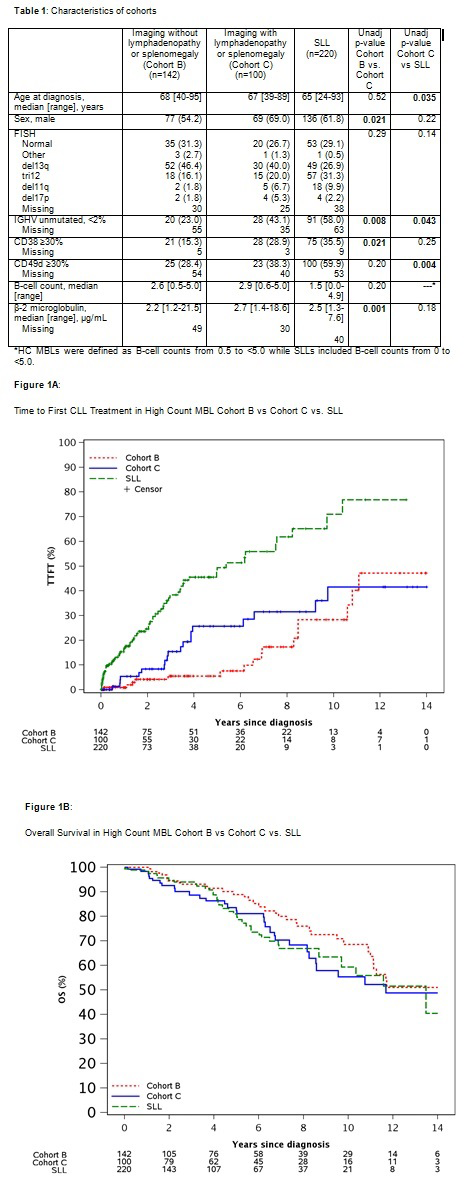
657 individuals with high-count MBL with a median follow-up of 6.7 years were identified: 415 (63%) individuals were in Cohort A, 142 (22%) in Cohort B, and 100 in (15%) Cohort C. Compared to Cohort B, individuals in Cohort C were more likely to have unmutated IGHV, high expression of CD38, and higher beta-2 microglobulin level (Table 1). SLL patients were younger, had higher rate of unmutated IGHV, and had high expression of CD49d compared to MBL individuals in Cohort C (Table 1). There was suggestive evidence of a difference in TFT between Cohorts B and C (estimated 10-year treatment rate 28.3% vs. 41.6%; p=0.16; HR 1.62 95% CI 0.84-3.15). TFT was significantly shorter in SLL patients compared to those with Cohort C (estimated 10-year treatment rate 71% vs. 41.6%, respectively, P<0.001; Figure 1A). There was a trend toward shorter OS in Cohort C compared to Cohort B (estimated 10-year OS rate 53.7% vs. 64.2%, respectively, P=0.09; Figure 1B). In contrast, there was no difference in OS among MBL individuals in Cohort C compared to those with SLL (estimated 10-year OS rate 56.9% vs. 62.6%, P=0.72).
Summary/Conclusion:
Our findings demonstrate that individuals with MBL who have incidentally identified lymphadenopathy/splenomegaly at diagnosis are more likely to have an unfavorable risk profile, shorter TFT and OS compared to patients who did not have lymphadenopathy/splenomegaly at MBL diagnosis. Additionally, these individuals have a longer TFT but similar OS compared to SLL patients seen during the same time interval. If externally validated, these findings have significant implications for individuals with newly diagnosed MBL.
Ding:Merck: Research Funding; DTRM Biopharma: Research Funding. Kenderian:Novartis: Patents & Royalties, Research Funding; Tolero: Research Funding; Humanigen: Other: Scientific advisory board , Patents & Royalties, Research Funding; Lentigen: Research Funding; Morphosys: Research Funding; Kite/Gilead: Research Funding. Kay:MorphoSys: Other: Data Safety Monitoring Board; Infinity Pharmaceuticals: Other: DSMB; Celgene: Other: Data Safety Monitoring Board; Agios: Other: DSMB. Parikh:AstraZeneca: Honoraria, Research Funding; Janssen: Research Funding; AbbVie: Honoraria, Research Funding; Acerta Pharma: Research Funding; Ascentage Pharma: Research Funding; Genentech: Honoraria; Pharmacyclics: Honoraria, Research Funding; MorphoSys: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal