Introduction: Germline RUNX1 mutations/deletions result in a Familial Platelet Disorder with propensity to Myeloid Malignancy (FPDMM); an autosomal dominant condition characterized by thrombocytopenia, qualitative platelet defects, and myeloid clonal evolution (MDS and AML). The advent of next generation sequencing (NGS) has allowed detection of clonal cytopenias of unclear significance (CCUS) prior to bone marrow (BM) morphological changes. Somatic RUNX1 mutations (RUNX1MT) can be seen in myeloid malignances, including MDS/MPN overlap syndromes, with an indeterminate prognostic impact. We carried out this study to define the landscape of clonal evolution in FPDMM patients and to compare germline RUNX1MT with somatic mutations.
Methods After IRB approval, families with RUNX1MT FPDMM (detected by germline sequencing and deletion/duplication assays) and patients with 2016, WHO-defined MDS/MPN overlap syndromes (except JMML) were included in the study. Index patients with FPDMM underwent BM biopsies and in select cases NGS at diagnosis and then as indicated for follow up, or at disease progression. All MDS/MPN overlap syndrome patients underwent a 29-gene panel NGS assay on BM samples obtained at diagnosis by previously described methods (Patnaik et al., BCJ 2016).
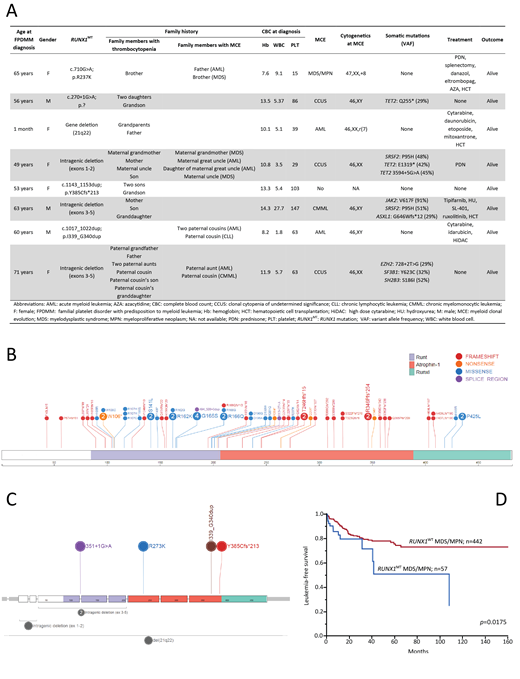
Results Eight index patients with FPDMM were included; median age at diagnosis 58 (range, 0-71) years, 3 (38%) males (Figure A). Four (50%) had intragenic deletions involving multiple exons in 3 cases and 2 nucleotides in 1 case, 1 (13%) each had missense, frameshift and splice site mutations, while 1 (13%) patient had a whole gene deletion (Figure C). Notably, in 4 (100%) of 4 patients with large deletions, NGS testing was negative for RUNX1 abnormalities.
Four (50%) index patients presented with a myeloid neoplasm; JAK2, SRSF2, ASXL1 mutant CMML, MDS/MPN-U with trisomy 8, AML with del7q and normal karyotype AML (negative NGS). Three (38%) index patients had CCUS at diagnosis with TET2 (67%) being the most common acquired mutation, and others including SRSF2, EZH2, and SF3B1. Cumulatively, myeloid clonal evolution was seen in 7 (88%) patients. Three (38%) index patients (AML-2, CMML-1) underwent allogeneic HCT, and at last follow up were in remission. Median age at myeloid clonal evolution was 61 (range, 4-71) years; median overall survival (OS) from date of myeloid clonal evolution was 12 (range, 7-53) months.
For the comparative analysis, 499 patients with MDS/MPN overlap syndromes were included; 382 (77%) with CMML, 48 (10%) with MDS/MPN-RS-T (MDS/MPN with ring sideroblasts and thrombocytosis), 17 (3%) with aCML (atypical CML), and 52 (10%) with MDS-MPN-U (unclassifiable). Median age at diagnosis was 71 (range, 18-99) years, and 329 (66%) were male. RUNX1MT were detected in 57 (11%) patients; 43 (11%) with CMML, 5 (30%) with aCML, and 9 (17%) with MDS-MPN-U. No RUNX1MT were found in MDS-MPN-RS-T. Overall, 62 RUNX1MT were identified in 57 patients, with 52 (39 CMML, 4 aCML, 9 MDS-MPN-U) and 5 (4 CMML, 1 aCML) patients harboring 1 and 2 RUNX1MT, respectively. RUNX1MT spanned the entire coding sequence without specific hotspots (Figure B): 27 (44%) were missense, 2 (3%) involved the splicing sites, 5 (8%) were nonsense and 28 (45%) were frameshift. There was only one RUNX1MT (R273K), seen in both germline and somatic patients (Figures B and C).
Compared to wild type patients, RUNX1MT patients with MDS/MPN overlap syndromes had significantly lower platelet counts (median 72 vs 117 x109/L, p<0.0001), without any other phenotypic differences. In the somatic RUNX1MT cohort, co-occurring mutations included ASXL1 (54%), SRSF2 (54%), TET2 (49%), and NRAS (28%), with NRAS (28% vs 13%, p=0.0028), and SRSF2 (54% vs 39%, p=0.0218) mutations clustering with RUNX1MT. Median OS of the entire cohort was 29 (range, 0-170) months. On univariate analysis, while there was a trend for an inferior OS in RUNX1MTvs wild type patients (20 vs 29 months, p=0.0523), the leukemia-free survival was clearly inferior (p=0.0175, Figure D).
ConclusionRUNX1MT FPDMM is a germline predisposition syndrome with a high rate of myeloid clonal evolution (88%), including clonal cytopenias (38%). Germline RUNX1MT have minimal overlap with somatic mutations seen in myeloid malignancies, with NGS testing often missing RUNX1 deletions. Somatic RUNX1MT in myeloid malignancies are significantly associated with thrombocytopenia and an inferior leukemia-free survival.
Patnaik:Stem Line Pharmaceuticals.: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal