Background
The oral Janus kinase (JAK) 1/JAK2 inhibitor RUX has been shown to induce a reduction in bone marrow (BM) fibrosis in patients (pts) with myelofibrosis (MF) compared with placebo. MF is characterized by clonal proliferation of hematopoietic progenitor cells (HPCs) and amplification of cytokine-producing megakaryocytes (MEGs) and macrophages (MACs). Evidence suggests that the pro-inflammatory microenvironment, fostered by the hematopoietic clone, results in BM stromal alterations (including BM fibrosis and osteosclerosis), which can, in turn, influence the hematopoietic niche. The objective of this analysis was to evaluate BM changes in order to characterize the long-term effects of RUX on BM stromal alterations, cytokine-producing cells (ie, MEGs and MACs), and plasma cells (surrogates of inflammation) in a cohort of pts with intermediate-2 or high-risk primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF who were enrolled in the phase 3 COMFORT-I study.
Methods
This analysis included 57 pts (36 originally randomized to RUX, 21 crossover [CO] from placebo). All pts were required to have a baseline (BL) BM biopsy and ≥1 subsequent BM observation. CO pts were re-baselined; if CO occurred at <36 wks or ≥36 wks, the Week 0 BM or Week 48 BM, respectively, was used as the BL observation. For fibrosis and osteosclerosis, sections were stained with standard procedures. Specific immunohistochemical (IHC) stains were used to assess MEGs (CD61); plasma cells (MUM1); and activated MACs, including CD68 to identify M1 and anti-inflammatory M2 subtypes, and CD163, a very specific marker for the M2 subtype. Evaluations included IHC and morphometric assessment of CD34+ HPCs. European consensus guidelines were used to grade BM fibrosis and cellularity (Haematologica. 2005;90:1128-1132). Each parameter was graded, by consensus, based on independent review by 3 expert pathologists. A 0-3 grading system was used for fibrosis, osteosclerosis, plasma cells, MEG clustering/atypia, and CD68/163 MACs. Changes from BL to last BM observation are reported. These changes were categorized as improved, stable, or worsened. Improvement/worsening was defined as ≥1 grade reduction/increase from BL or a change in abnormal/normal status. Improvement was assessed in pts with BL values 1-3 or abnormal, stability in pts with BL values 0-2 or normal, and worsening in pts with BL values 0-2 or normal.
Results
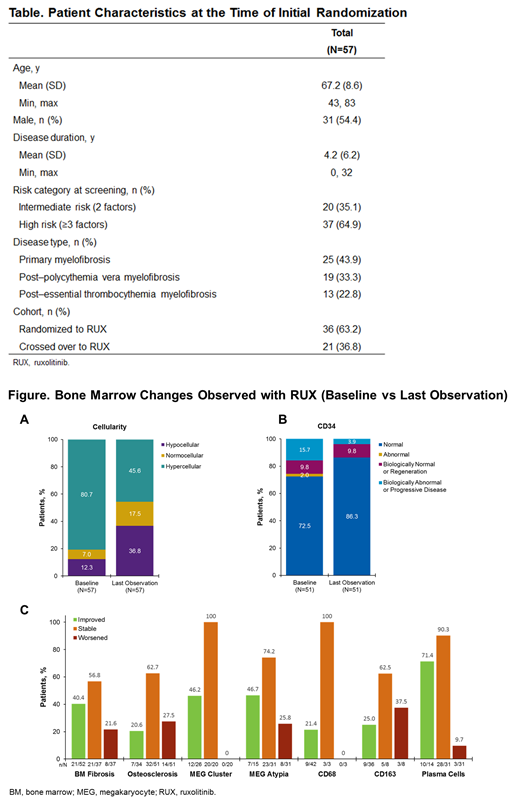
Pt characteristics are summarized in the Table. With respect to age-adjusted cellularity at BL, most marrows were hypercellular, with a notable increase in the proportion of normocellular and hypocellular marrows by the last observation (Figure). In most pts, BM fibrosis was either stable or improved; 21.6% of pts had worsening fibrosis. Similarly, the majority of pts had stable (62.7%) or improved (20.6%) osteosclerosis at the last observation compared with BL. Assessments of megakaryopoiesis revealed stabilization or improvement in MEG clustering in all pts; MEG atypia was improved or stable in the majority of pts. Similarly, RUX resulted in normalization of CD68+ and CD163+ MACs in 21.4% and 25.0% of pts, respectively. The proportion of pts with normal CD34+ clustering/frequency increased from 72.5% at BL to 86.3% at the last visit.
With respect to evidence of decreased inflammation in the BM microenvironment, the majority of pts (71.4%) with abnormal plasma cells at BL had a normal level of plasma cells by the last observation, and only 9.7% had evidence of worsening.
Conclusions
These results extend previous observations on the effect of RUX on the BM in pts with MF. RUX treatment resulted in improvements in the HPCs, atypical MEGs, and activated MACs that are classically thought to produce the inflammatory cytokines that drive BM stromal alterations. These improvements in myeloproliferation were associated with improvement/stabilization of BM fibrosis and sclerosis in the majority of pts. There were also directional improvements in BM plasma cells, a surrogate of inflammation in the BM microenvironment. The MF phenotype is derived from clonal myeloproliferation and a secondary inflammatory state, which results in BM stromal alterations and a constellation of symptoms. The disease-modifying properties of RUX are likely attributable to its ability to address not only myeloproliferation through inhibition of JAK2, but also the secondary inflammatory state through inhibition of JAK1.
Kvasnicka:Novartis: Honoraria, Research Funding; Takeda: Honoraria; Incyte: Honoraria, Research Funding. Thiele:Sanofi: Consultancy, Honoraria, Other: Remuneration; Incyte: Consultancy, Honoraria, Other: Remuneration, Research Funding; AOP Orphan Pharmaceuticals: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Other: Remuneration, Research Funding; Shire: Research Funding. Bueso-Ramos:Incyte: Consultancy. Colucci:Incyte: Employment, Equity Ownership. Paranagama:Incyte: Employment, Equity Ownership. Verstovsek:Genetech: Research Funding; Blueprint Medicines Corp: Research Funding; Gilead: Research Funding; Promedior: Research Funding; CTI BioPharma Corp: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Research Funding; Pharma Essentia: Research Funding; Astrazeneca: Research Funding; Ital Pharma: Research Funding; Protaganist Therapeutics: Research Funding; Constellation: Consultancy; Pragmatist: Consultancy; Incyte: Research Funding; Roche: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal