Background:
There are unresolved questions regarding the association between leukocytosis and risk of thrombosis and disease evolution in polycythemia vera (PV). Published literature on this topic suffers from several key limitations: first "leukocytosis" is often defined as a value measured at a single time point (usually diagnosis) in patient follow-up. Secondly, most do not employ any form of time-dependent modeling. These analyses may be poor representations of how a clinician observes and reacts to this lab value over time, providing little insight into how longitudinal lab data or leukocyte normalization may associate with thrombotic or evolutionary hazard. Valuable information about cumulative exposure to leukocytosis and information related to the benefit of leukoreduction may be discarded. To address this knowledge gap and introduce statistical methodology that better utilizes repeated measures of hematologic lab data, we constructed a retrospective database of 520 PV patients from 10 academic institutions across the United States.
Methods:
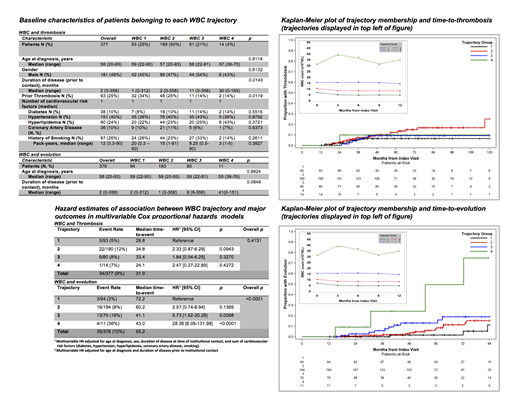
Patients were included in the database if they were ≥18 years of age at diagnosis, met 2016 WHO diagnostic criteria for PV, had ≥ 3 visits with a hematologist at a participating site, and were seen within the past 10 years. We used group-based trajectory modeling (GBTM) to identify clusters of patients following distinct lab value trajectories over time. Four WBC trajectories were identified representing patients with stable WBC at 5, 10, 15 x 109 /L, and one trajectory oscillating around 35 x 109 /L (WBC 1-4, respectively). Three hematocrit trajectories, representing patients with initially decreasing then stable hematocrits at 35%, 43%, and 47%, and three stable platelet trajectories at 125, 300, and 600 x 109 /L were also identified.
Multivariable Cox proportional hazards methods were used to examine the associations between trajectory membership for all three hematologic indices and thrombosis (in an evaluable subset of 377 patients; 34 events) and evolution to myelofibrosis, myelodysplastic syndrome, or acute myeloid leukemia (in a 378-patient subset; 35 events). All models for thrombosis were adjusted for age at diagnosis, gender, duration of disease prior to institutional contact, history of prior thrombotic event, and number of relevant cardiovascular risk factors. All models concerning disease evolution were adjusted for age at diagnosis and duration of disease prior to institutional contact.
Results:
In our Cox-proportional hazards model, no significant association between WBC trajectory and thrombosis was found (overall p = 0.4131). There was, however, a significant association between WBC trajectory and hazard of disease evolution (overall p < 0.0001). Membership in WBC trajectory 2 (stable at 10 x 109 /L) did not significantly increase hazard of evolution (p = 0.1385), whereas membership in WBC trajectory 3 (stable at 15 x 109 /L) increased hazard of evolution by 5.73-fold (95% CI 1.62 - 20.28, p = 0.0068), and membership in WBC trajectory 4 (oscillation at 35 x 109 /L) increased hazard of evolution by 28.36-fold (95% CI 6.09 - 131.98, p < 0.0001). There was no statistically significant association between hematocrit trajectory and thrombosis (p = 0.2391), hematocrit trajectory and disease evolution (p = 0.5525), platelet trajectory and thrombosis (p = 0.9308), or platelet trajectory and disease evolution (p = 0.1948).
Conclusions:
In subsets of patients from a large multi-center US database, GBTM and multivariable Cox modeling was used to assess the associations between longitudinal lab data and disease outcomes. Interestingly, WBC trajectory was not associated with increased thrombotic hazard. Although our study does not serve as conclusive evidence that leukocytosis is not associated with thrombosis, it reinforces the uncertainty of leukocytosis as a thrombotic risk factor. The hazard of disease evolution significantly increased with increasing WBC trajectory in a stepwise manner, suggesting that persistent leukocytosis may serve as an important marker of disease progression. A prospective trial of leukocyte control in otherwise uncontrolled patients with an endpoint of disease evolution is warranted. Results of such a trial could have broad impact on the management of low-risk patients with persistent leukocytosis.
Podoltsev:Genentech: Research Funding; Celgene: Other: Grant funding, Research Funding; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AI Therapeutics: Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Research Funding; Astellas Pharma: Research Funding; Daiichi Sankyo: Research Funding; Sunesis Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Research Funding; Pfizer: Research Funding; Astex Pharmaceuticals: Research Funding; CTI Biopharma: Research Funding; Samus Therapeutics: Research Funding; Arog Pharmaceuticals: Research Funding; Kartos Therapeutics: Research Funding. Gotlib:Pharmacyclics: Research Funding; Promedior: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Deceiphera: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Allakos: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Heaney:Incyte: Consultancy, Research Funding; Celgene: Research Funding; Novartis: Consultancy; CTI: Research Funding; Constellation: Research Funding; BMS: Research Funding; Partner Therapeutics: Consultancy; AbbVie: Consultancy; Roche: Consultancy, Research Funding; Deciphera: Research Funding; Blueprint: Research Funding. Kuykendall:Celgene: Honoraria; Janssen: Consultancy; Abbvie: Honoraria; Incyte: Honoraria, Speakers Bureau. O'Connell:Pfizer: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Astex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees. Shammo:Incyte: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Alexion: Consultancy, Honoraria, Research Funding, Speakers Bureau; Apellis: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Speakers Bureau; Astex Pharma: Research Funding; Novartis: Consultancy, Honoraria; Otsuka: Consultancy, Honoraria; CTI Pharma: Research Funding; Onconova: Research Funding. Fleischman:incyte: Speakers Bureau. Scherber:Gilead: Consultancy; Incyte: Consultancy; Blueprint: Other: Ad board. Mesa:Celgene: Research Funding; Incyte: Research Funding; Promedior: Research Funding; Samus: Research Funding; Sierra Onc: Consultancy; Novartis: Consultancy; La Jolla Pharma: Consultancy; CTI Biopharma: Research Funding; Genotech: Research Funding; AbbVie: Research Funding. Yacoub:Dynavax: Equity Ownership; Cara: Equity Ownership; Ardelyx: Equity Ownership; Incyte: Consultancy, Honoraria, Speakers Bureau; Seattle Genetics: Honoraria, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Agios: Speakers Bureau; Hylapharm: Equity Ownership. Hoffman:Merus: Research Funding. Mascarenhas:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmaessentia: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merus: Research Funding; Janssen: Research Funding; Promedior: Research Funding; Merck: Research Funding; Roche: Consultancy, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal