Introduction: CD19-specific chimeric antigen receptor (CAR) T-cell therapy is FDA approved in patients with relapsed or refractory large B-cell lymphomas. While 35-40% of patients may achieve a durable complete response (CR), the toxicity incurred with CAR-T therapy could impact the ability to receive subsequent treatment in those who progress after CAR-T infusion. Our prior data suggested that patients who experienced early progression had inferior overall survival. We now update our results and evaluate the impact of laboratory abnormalities and comorbidities at the time of progression on overall survival.
Methods: Adults with large B-cell lymphomas who received CD19-specific CAR T-cells at the University of Washington/Seattle Cancer Care Alliance were included. Patients who received CAR T-cell therapy with additional concurrent protocol-specified therapy were excluded. Those who exhibited progressive disease (PD) or persistent lymphoma after CAR T-cell therapy were the focus of this study. We defined patients who progressed or received additional lymphoma directed therapy after last CAR-T cell infusion as early PD, with all other patients defined as late PD. We collected laboratory data closest to the date of progression. We defined an absolute neutrophil count < 1000, platelet count < 75K, Creatinine > upper limit of normal (ULN), INR > ULN, AST/ALT > 2.5x ULN, total bilirubin > ULN, and LDH > ULN as abnormal. Primary endpoint of this analysis was overall survival (OS) landmarked to date of progression. Secondary endpoints include sub-group analyses based on early PD as well as lab abnormalities at the time of progression. A multi-variate analysis with select baseline and progression variables was also performed.
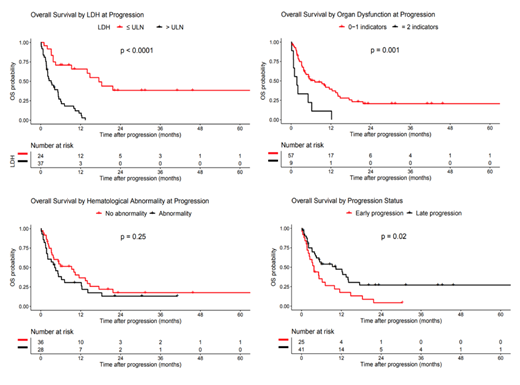
Results: We identified 66 patients who met the above criteria. Median follow up for the entire cohort is 30.4 months (range 0.1-64 months) by reverse KM method. Median time from last planned CAR infusion to progression was 43.5 days (range 11-658). Median OS of the entire cohort was 5.43 months (95% CI 3.75-12.2). 25 (38%) patients experienced early PD, which was associated with inferior OS (median 3.75 vs. 10.4 months, P=0.02). LDH > ULN at the time of progression defined a group with inferior outcomes (median OS 3.16 vs. 17.5 months, P<0.0001). Patients with at least one hematologic abnormality (ANC <1000 and/or platelets < 75K) had similar outcomes to those with higher values (median OS 4.18 vs 9.28 months, P=0.25). However, when we incorporated measurements of organ function, we found that patients with >1 indicator of hematologic and/or organ dysfunction (excluding LDH) at the time of progression had worse outcomes compared to those with one or fewer abnormalities (median OS 1.74 vs. 7.14 months, P=0.001). Multivariate analysis identified pre-CAR IPI score 4-5 (HR 6.33, 95% CI 1.97-20.36), LDH > ULN at progression (7.01, 95% CI 2.89-17.013), and abnormal creatinine at progression (5.32, 95% CI 1.71-16.53), as factors associated with increased risk of death.
Conclusions: Patients with PD post CD19-specific CAR T-cell therapy, particularly those with early PD, elevated LDH, or renal failure experience extremely poor outcomes. These data can inform discussion of prognosis for patients who progress after CAR T-cell therapy and may predict which patients may benefit from additional anti-lymphoma therapy.
Lynch:Johnson Graffe Keay Moniz & Wick LLP: Consultancy; Juno Therapeutics: Research Funding; Takeda Pharmaceuticals: Research Funding; T.G. Therapeutics: Research Funding; Incyte Corporation: Research Funding; Rhizen Pharmaceuticals S.A: Research Funding. Maloney:A2 Biotherapeutics: Honoraria, Other: Stock options ; Celgene,Kite Pharma: Honoraria, Research Funding; Juno Therapeutics: Honoraria, Patents & Royalties: patients pending , Research Funding; BioLine RX, Gilead,Genentech,Novartis: Honoraria. Turtle:Nektar Therapeutics: Other: Ad hoc advisory board member, Research Funding; Juno Therapeutics: Patents & Royalties: Co-inventor with staff from Juno Therapeutics; pending, Research Funding; Eureka Therapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Caribou Biosciences: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Novartis: Other: Ad hoc advisory board member; Precision Biosciences: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; T-CURX: Membership on an entity's Board of Directors or advisory committees; Allogene: Other: Ad hoc advisory board member; Kite/Gilead: Other: Ad hoc advisory board member; Humanigen: Other: Ad hoc advisory board member. Smith:Portola Pharmaceuticals: Research Funding; Pharmacyclics: Research Funding; Ignyta (spouse): Research Funding; Genentech: Research Funding; Denovo Biopharma: Research Funding; Ayala (spouse): Research Funding; Bristol-Myers Squibb (spouse): Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta Pharma BV: Research Funding; Merck Sharp & Dohme Corp: Consultancy, Research Funding; Seattle Genetics: Research Funding; Incyte Corporation: Research Funding. Shadman:TG Therapeutic: Research Funding; Mustang Bio: Research Funding; Atara Biotherapeutics: Consultancy; Pharmacyclics: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Sunesis: Research Funding; Verastem: Consultancy; Astra Zeneca: Consultancy; ADC Therapeutics: Consultancy; Sound Biologics: Consultancy; Celgene: Research Funding; Gilead: Consultancy, Research Funding; BeiGene: Research Funding; Acerta Pharma: Research Funding. Ujjani:Pharmacyclics: Honoraria; Atara: Consultancy; Gilead: Consultancy; Genentech: Honoraria; Astrazeneca: Consultancy; AbbVie: Honoraria, Research Funding; PCYC: Research Funding. Cassaday:Amgen: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Kite/Gilead: Research Funding; Merck: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Other: Spouse's disclosure: employment, stock and other ownership interests. Till:Mustang Bio: Patents & Royalties, Research Funding. Shustov:Seattle Genetics, Inc.: Research Funding. Gopal:Seattle Genetics, Pfizer, Janssen, Gilead, Sanofi, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Takeda, Compliment, Asana Bio, and Incyte.: Consultancy; Seattle Genetics, Pfizer, Janssen, Gilead, Sanofi, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Takeda, Compliment, Asana Bio, and Incyte: Honoraria; Teva, Bristol-Myers Squibb, Merck, Takeda, Seattle Genetics, Pfizer, Janssen, Takeda, and Effector: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal