Background: We have previously shown that CMR predicts better outcomes in Ph+ ALL. The lack of achievement of CMR and particularly major molecular response (MMR) at 3 months may confer poor outcomes. We sought to investigate the outcomes of pts who did not achieve CMR at 3 months as best response in terms of progression free survival (PFS) and overall survival (OS), and the role of allogeneic stem cell transplant (ASCT) in this population.
Methods: We reviewed 204 pts with newly diagnosed Ph+ ALL treated at our institution between January 2001 and June 2019 with the combination of Hyper-CVAD plus tyrosine kinase inhibitors (TKI); dasatinib (n=88, 43%), ponatinib (n=72, 35%) and imatinib (n= 44, 22%). PFS was defined from the start of therapy to relapse or death. OS was defined from diagnosis to death or last follow-up. Backward multivariate Cox regression was used to identify prognostic factors for PFS and OS after variable selection at a p-value cutoff of 0.200. Time to ASCT was handled as a time-dependent variable. Survival curves were estimated by Kaplan-Meier method. Landmark analysis at the median time to ASCT was analyzed to evaluate the impact of ASCT.
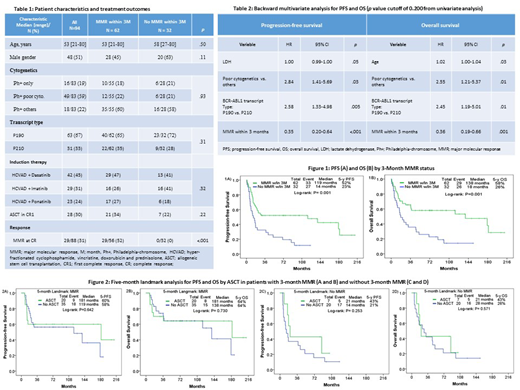
Results: We identified 94 pts (46%) who did not achieve 3-month CMR. Of pts treated with imatinib, 29 (66%) did not achieve 3-month CMR and 16 pts (36%) achieved 3-month MMR. Of pts treated with dasatinib, 42 (48%) did not achieve 3-month CMR and 29 pts (33%) achieved 3-month MMR. Of pts treated with ponatinib, 23 (32%) did not achieve 3-month CMR and 17 pts (24%) achieved 3-month MMR. Patient characteristics are summarized in table 1. Median age was 54 years (range: 21-80). The TKI administered was dasatinib, imatinib and ponatinib in 42 (45%), 29 (31%) and 23 (24%) pts, respectively. Overall, ASCT was performed in 28 pts (30%); 21 out of 62 pts (34%) with 3-month MMR, and 7 out of 32 pts (22%) who did not achieve MMR, within a median time of 5 months (range, 2.3-12.3). After a median follow-up of 97 months, median PFS was 21 months and median OS was 46 months. There was no difference in survival by TKI choice. The 5-year PFS and OS rates were 52% and 23% (p=0.001) (Figure 1A), and 58% and 26% (p=0.001) (Figure 1B) for pts with and without 3-month MMR, respectively.
In multivariate analysis (table 2), 3-month MMR predicted longer PFS (p<0.001; hazard ratio [HR], 0.35; 95% confidence interval [CI], 0.20-0.64), and OS (p=0.001; HR 0.35; 95% CI, 0.19-0.66). In contrast, clonal evolution and transcript type were prognostic for shorter PFS (p=0.03; HR, 2.84; 95% CI, 1.41-5.69, and p=0.005; HR, 2.58; 95% CI, 1.33-4.98, respectively) and OS (p=0.01; HR 2.556; 95% CI, 1.21-5.37, and p=0.014; HR 2.45; 95% CI, 1.19-5.01, respectively). Additionally, older age was predictive for poor OS (p=0.03; HR, 1.02; 95% CI, 1.00-1.04). ASCT was not predictive for PFS nor OS.
A 5-month landmark analysis was performed to assess the effect of ASCT on survival by 3-month MMR status. In pts who achieved 3-month MMR, 5-year PFS and OS rates were 60% and 58% (p=0.64) (Figure 2A) and 64% and 64% (p=0.73) (Figure 2B) in pts with and without ASCT, respectively. In contrast, among pts who did not achieve a 3-month MMR, there was a tendency for better PFS (5-PFS, 43% vs 21%; p=0.25) (Figure 2C) and OS (5-year OS, 43% vs. 26%; p=0.57) (Figure 2D) in favor of ASCT.
Conclusions: The lack of achievement of MMR at 3 months predicted worse outcomes in Ph+ ALL. Higher survival rates were seen when ASCT was performed in pts who do not achieve 3-month MMR. Innovative targeted strategies aimed to eradicate minimal residual disease are needed to further improve long term outcomes.
Kantarjian:Cyclacel: Research Funding; AbbVie: Honoraria, Research Funding; BMS: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Research Funding; Jazz Pharma: Research Funding; Astex: Research Funding; Daiichi-Sankyo: Research Funding; Takeda: Honoraria; Amgen: Honoraria, Research Funding; Ariad: Research Funding; Immunogen: Research Funding; Agios: Honoraria, Research Funding; Novartis: Research Funding. Sasaki:Otsuka: Honoraria; Pfizer: Consultancy. Short:AstraZeneca: Consultancy; Takeda Oncology: Consultancy, Research Funding; Amgen: Honoraria. Khoury:Stemline Therapeutics: Research Funding; Angle: Research Funding; Kiromic: Research Funding. Champlin:Actinium: Consultancy; Johnson and Johnson: Consultancy; Sanofi-Genzyme: Research Funding. Kebriaei:Amgen: Research Funding; Jazz: Consultancy; Pfizer: Honoraria; Kite: Honoraria. Cortes:Forma Therapeutics: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; BiolineRx: Consultancy; Takeda: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Research Funding. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Kadia:Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Bioline RX: Research Funding; BMS: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding. Borthakur:AstraZeneca: Research Funding; NKarta: Consultancy; Cyclacel: Research Funding; GSK: Research Funding; Xbiotech USA: Research Funding; Oncoceutics: Research Funding; Janssen: Research Funding; Incyte: Research Funding; Oncoceutics, Inc.: Research Funding; Eli Lilly and Co.: Research Funding; BMS: Research Funding; Tetralogic Pharmaceuticals: Research Funding; Strategia Therapeutics: Research Funding; Polaris: Research Funding; Arvinas: Research Funding; Merck: Research Funding; Cantargia AB: Research Funding; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Argenx: Membership on an entity's Board of Directors or advisory committees; BioTheryX: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; AbbVie: Research Funding; Bayer Healthcare AG: Research Funding; PTC Therapeutics: Consultancy; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agensys: Research Funding; Eisai: Research Funding. Verstovsek:Roche: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding; Gilead: Research Funding; Promedior: Research Funding; CTI BioPharma Corp: Research Funding; Genetech: Research Funding; Blueprint Medicines Corp: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Research Funding; Pharma Essentia: Research Funding; Astrazeneca: Research Funding; Ital Pharma: Research Funding; Protaganist Therapeutics: Research Funding; Constellation: Consultancy; Pragmatist: Consultancy; Incyte: Research Funding. Daver:Agios: Consultancy; Hanmi Pharm Co., Ltd.: Research Funding; Genentech: Consultancy, Research Funding; Forty-Seven: Consultancy; Novartis: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; NOHLA: Research Funding; Glycomimetics: Research Funding; Otsuka: Consultancy; Celgene: Consultancy; Immunogen: Consultancy, Research Funding; Jazz: Consultancy; Astellas: Consultancy; BMS: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Servier: Research Funding; Abbvie: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Jain:Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Cellectis: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Konopleva:F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Eli Lilly: Research Funding; Forty-Seven: Consultancy, Honoraria; Calithera: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding. O'Brien:Eisai: Consultancy; Gilead: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Kite: Research Funding; GlaxoSmithKline: Consultancy; Regeneron: Research Funding; TG Therapeutics: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Verastem: Consultancy; Vaniam Group LLC: Consultancy; Celgene: Consultancy; Astellas: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Acerta: Research Funding; Alexion: Consultancy; Amgen: Consultancy; Aptose Biosciences, Inc: Consultancy. Ravandi:Cyclacel LTD: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Macrogenix: Consultancy, Research Funding; Menarini Ricerche: Research Funding; Selvita: Research Funding; Xencor: Consultancy, Research Funding. Jabbour:Adaptive: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Cyclacel LTD: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal