Background: There remains an unmet need for novel therapeutic strategies for relapsed or refractory (R/R) acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LLy). Venetoclax (Ven) is a highly selective BCL-2 inhibitor with established efficacy in adults with hematologic malignancies. Navitoclax (Nav) is a BCL-2/BCL-XL/BCL-W inhibitor with promising effects, but prolonged thrombocytopenia limits its continuous use at higher doses (J Clin Oncol. 2012;30:488). In a previous report of 32 patients (pts) with ALL, Ven+Nav was well tolerated without unexpected toxicity and with promising preliminary efficacy (EHA 2019. Abstr #PS940). Here we report updated outcomes for 36 pts with ALL or LLy treated with Ven+Nav and chemotherapy.
Methods: This phase 1, multicenter, open-label, dose escalation study enrolled pts aged ≥4 years with R/R ALL or LLy (NCT03181126). Pts received the weight-adjusted equivalent of 200 mg Ven on day 1 and 400 mg equivalent daily thereafter. From day 3 onward, oral daily Nav was administered at 25, 50, or 100 mg for pts weighing ≥45 kg or at 25 or 50 mg for pts weighing 20 to <45 kg according to Bayesian optimal interval design. Pts could receive 2 cycles of chemotherapy with asparaginase, vincristine, and dexamethasone and additional cycles at the investigators' discretion. Disease assessment by flow cytometry occurred on days 8 and 36 and as clinically indicated. Minimal residual disease (MRD) evaluation (<10-4 cutoff for undetectable MRD) was performed at time of disease assessment if clinically indicated.
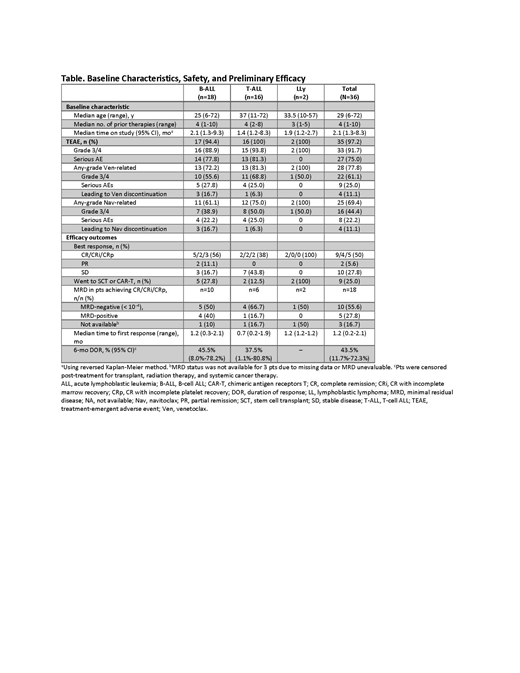
Results: As of May 6, 2019, 36 pts have been enrolled with B-cell ALL (B-ALL, n=18), T-cell ALL (T-ALL, n=16), or LLy (n=2). The median age was 29 years (range, 6-72 years), including 7 pediatric pts (Table), and pts had a median of 4 prior therapies (range, 1-10) including 5 (14%) with prior stem cell transplant (SCT) and 6 (17%) with prior CAR-T therapy. Median time on study was 2.1 months (range, 1.3 - 8.3 months). The most common treatment-emergent adverse events (AEs) were nausea (47%), vomiting (42%), diarrhea (42%), hypokalemia (42%), abdominal pain (36%), febrile neutropenia (33%), alanine aminotransferase increase (31%), and neutropenia (31%). The only grade 3/4 non-hematologic AE related to Ven occurring in more than 1 pt was vomiting (3 [8%]); only hematologic events related to Nav occurred in >1 pt. Dose-limiting toxicities included ischemic bowel and delayed count recovery (n=1 each, 100 mg Nav, ≥45 kg); drug-induced hepatitis (n=1, 50 mg Nav, ≥45 kg); and delayed count recovery (n=1, 25 mg, <45 kg). Three pts had fatal AEs not related to disease progression that included 2 cases of sepsis and 1 case of cardiac arrest in a pt with known underlying arrhythmia. Four other pts died in the setting of disease progression (ischemic bowel, sepsis, respiratory failure, and other) while receiving study drugs or within 30 days of stopping study drugs. The overall response rate (ORR) was 56% (20/36) in the total population, with best responses of complete response (CR)/CR with incomplete marrow recovery (CRi)/CR with incomplete platelet recovery (CRp) in 18 pts (50%; Table). In pediatric patients, the ORR was 86% (6/7) with best responses of CR/CRi/CRp in 5 pts (71%). Of the 18 pts with CR/CRi/CRp, 10 (56%) had undetectable MRD. Twenty-five percent of pts went on to SCT or CAR-T after achieving good response. There was no significant pharmacokinetic interaction between venetoclax and navitoclax. Preliminary assessment of mitochondrial priming by BH3 profiling confirmed that pt ALL cells showed dependence on BCL-XL and/or BCL-2. Genomic analyses are underway.
Conclusions:Ven+Nav in combination with chemotherapy is well tolerated, with few discontinuations or dose reductions from AEs in pts with R/R ALL or LLy. The preliminary efficacy of Ven+Nav was promising in this heavily pretreated population of pts including those with prior SCT or CAR-T, with high rates of CR/CRi/CRp, and 10/18 (56%) with undetectable MRD. Additional correlative biomarker analyses are ongoing and will be presented.
Stock:Kite, a Gilead Company: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; UpToDate: Honoraria; Research to Practice: Honoraria. Jabbour:Adaptive: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Bajel:AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: travel funding. Rubnitz:AbbVie: Research Funding. Leonard:Amgen: Research Funding. Mullighan:Illumina: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored travel; Pfizer: Honoraria, Other: speaker, sponsored travel, Research Funding; AbbVie: Research Funding; Loxo Oncology: Research Funding; Amgen: Honoraria, Other: speaker, sponsored travel. Khaw:Amgen: Other: travel accommodations, Research Funding; Bristol Myers Squibb: Research Funding; Jazz Pharmaceuticals: Research Funding; AbbVie: Research Funding; Novartis: Other: travel accommodations. Opferman:AbbVie: Research Funding. Salem:AbbVie: Employment, Other: Stock/stock options. Schmidt:AbbVie: Employment, Other: stock or options. Tong:AbbVie: Employment, Other: stock or options. Zhou:AbbVie: Employment, Other: Stock or options. Ross:AbbVie: Employment, Other: Stock/stock options. Bensman:AbbVie: Employment, Other: stock or options. Jacobson:AbbVie: Employment, Other: Stock or options. Alexander:AbbVie: Other: travel funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal