Introduction
Chimeric antigen receptor (CAR) T cell therapy targeting CD19 has demonstrated high success for B-cell acute lymphoblastic leukemia (B-ALL). Despite the initial high complete remission (CR) rate, about half of patients (pts) relapse at 1 year. CD19 antigen loss was observed in a significant number of relapsed patients. CD22 is another leukemic marker that often expressed on the surface of CD19- relapsed B-ALL blasts. We have developed a bispecific CAR construct targeting CD19 and CD22. Here we report results from a phase Ⅰ clinical trial of CD19/CD22 (GC022) dual CAR-T to evaluate the safety and feasibility of treating patients with relapsed/refractory B-ALL.
Methods
The CD19/CD22 dual CAR-T cells were manufactured in a cGMP facility. Patients' peripheral blood (PB) mononuclear cells were first collected, and CD3+ T cells were separated. The cells were then transfected by lentivirus encoded with CD19 CD22 bispecific scFv sequences. The CAR-T cells produced in this way contained a 4-1BB co-stimulatory signal domain. The CAR-T cells were then cultured for 8-14 days until sufficient cells were harvested for infusion.
All pts received conditioning regimen of fludarabine and cyclophosphamide intravenously for 3 consecutive days with doses of 30 mg/m2/day and 250 mg/m2/day, respectively before a single infusion of CAR-T cells. The level of infused CAR-T cell proliferation in PB was analyzed by qPCR and flow cytometry. The primary end points were to evaluate feasibility and toxicity, and the secondary end points included disease response and engraftment/persistence of infused CD19/CD22 dual CAR-T cells.
Results
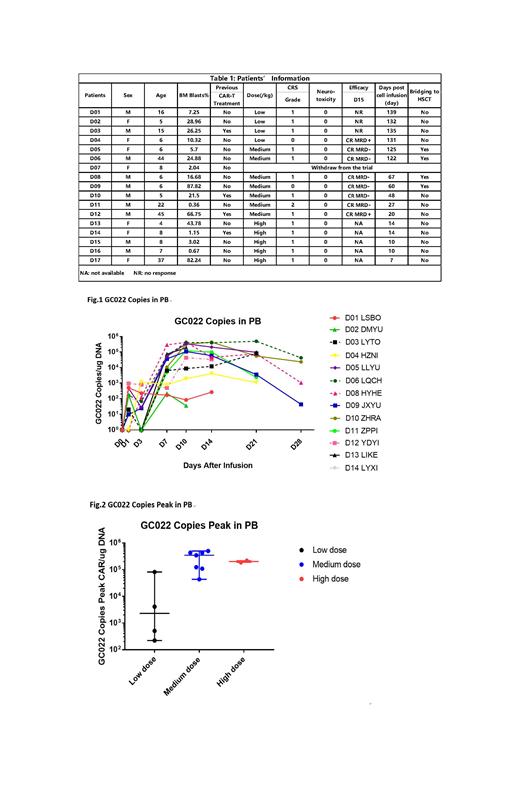
From Feb. 2019 to 23 July. 2019, 17 patients (pts) with relapsed/refractory B-ALL including 4 pts who previously treated with CD19 CAR-T cells were enrolled and pts were treated with CD19/CD22 dual CAR-T GC022 (US NIH Clinical#: NCT03825731). Four were adults, 13 pediatrics (age 1-45, Table 1). The median bone marrow (BM) blasts was 19.09 (0.36-87.82) %. Four patients received a low-dose (2.5-5×105/kg) dual CAR-T, 7 received a medium-dose (1-2.5×106/kg) and 5, a high-dose (3-5×106/kg). One patient withdrew immediately before CAR-T infusion due to his personal issue. Anti-leukemic efficacy was evaluated in 11/16 pts (5 pts have not yet reached D15). The 3/4 pts received low dose of GC022 had no response to treatment and 1 had MRD-positive CR. Seven patients who received medium dose achieved 100% CR on D15, highlighting the dose-dependent anti-leukemic activity. Six out of seven pts had MRD negative CR in this medium dose group. Five pts in high dose group have not reached the time for evaluation. No one relapsed with a median observation time of 60 (7-139) days. Cellular kinetic data was analyzed. Median peak of CAR-T copies was 1.09 (0.0022-4.98) x105 copy number/µg PB genomic DNA (Fig.1). The proliferation of medium or high dose groups was significantly better than the low dose group 3.47(0.43-4.98) x105 vs. 0.023(0.0022-0.81) x105(P=0.02) and 2.02(1.89-2.16) x105 vs. 0.023(0.0022-0.81) x105(P=0.004), (Fig.2). The peaks of IL-6, IFN-γ, IL-10, and CD25 were observed around day 7-10. Sixteen out of seventeen pts had grade 0-1 cytokine release syndrome (CRS) and only 1 patient experienced grade 2 CRS. None developed neurotoxicity.
Conclusion
Our study demonstrates safety and technical feasibility of CD19 and CD22 dual CAR-T in treating patients with CD19+CD22+ relapsed/refractory B-ALL. A low toxicity with dose-dependent high CR rate including pts who previously treated with CD19 CAR-T cells were observed. Longer observation time and more patients are needed to evaluate a beneficial advantage of the CD19/CD22 dual CAR-T over CD19 CAR-T product.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal