Background
Clinical outcomes of patients with Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia (Ph+ B-ALL) have improved with combination of tyrosine kinase inhibitors (TKIs) with intensive chemotherapies. However, patients with IKZF1 deletion (del) has been shown to manifest poor prognosis when treated with imatinib-based regimens (Martinelli et al. J Clin Oncol. 2009, Veer et al. Blood 2014). Prognostic significance of IKZF1 del in the context of more potent TKIs, such as dasatinib or ponatinib, is not known.
Methods
We studied 62 patients with previously untreated Ph+ B-ALL who were part of the phase II clinical trials of Hyper-CVAD alternating with high-dose methotrexate and cytarabine (Hyper-CVAD/MA) plus dasatinib (N = 27) or Hyper-CVAD/MA plus ponatinib (N = 35). Details of the treatment protocols have been previously published elsewhere (Ravandi et al. Blood 2010 and Jabbour et al. Lancet Haematol 2018). DNA from diagnostic bone marrow samples were analyzed by genome-wide SNP arrays using Infinium Omni2.5 array (Illumina, N = 39), Infinium CytoSNP-850K array (Illumina, N = 20), or by whole exome sequencing (N= 3) for copy number analyses. Somatic point mutations were also detected by Agilent's SureSelect Custom 295 gene panel with median 500x depth.
Results
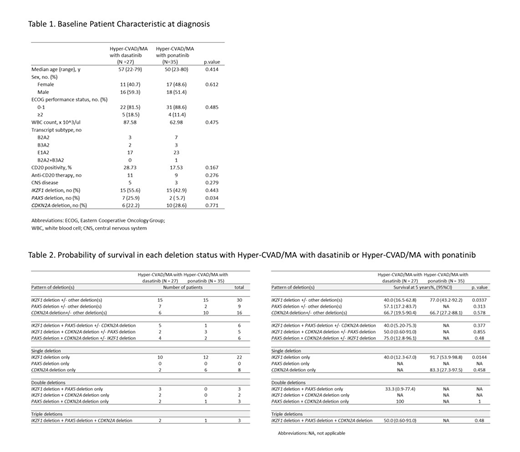
The baseline clinical characteristics of the 62 Ph+ B-ALL patients are described in Table 1. There were no significant differences in major clinical features between dasatinib- and ponatinib-treated groups. Overall, IKZF1 del, CDKN2A del, and PAX5 del were detected in 30 (48.3%), 16 (25.8%), and 9 (14.5%) patients, respectively. Frequency of IKZF1 and CDKN2A deletions were comparable between dasatinib- and ponatinib-treated groups, but PAX5 deletions were more frequently detected in dasatinib-treated group (dasatinib vs. ponatinib, 25.9% vs. 5.7%, P = 0.034) (Table 1). Somatic point mutations were rare in Ph+ B-ALL and 20 (32.3%) of the patients were found to have at least one point mutations. DNMT3A (N = 4) was most frequently detected among the cohort followed by CHEK2 (N = 2), EP300 (N = 2), SETD2 (N = 2), and TET2 (N = 2).
Irrespective of the treatment regimen, 100% of the patients attained complete remission (CR) after 1 cycle of therapy. 29 (48.3%) and 41 (68.3%) patients attained CR with negative minimal residual disease (MRD) with RT-PCR at 1 month and 3 months, respectively. Patients with IKZF1 del had significantly lower chance of attaining CR with negative MRD compared to IKZF1 wild type (WT) patients at 1 month (IKZF1 del vs. WT, 26.7% vs. 70.0% , P = 0.002). However, at 3 months, no difference of complete molecular response (CMR) was observed (IKZF1 del vs. WT, 71.0% vs. 65.5%, P = 0.783). Other deletions or point mutations did not affect MRD negative status.
With the median follow up duration of 59 months (95% confidence interval [CI]: 54-67), the median overall survival (OS) of the entire cohort was 80 months (95% CI: 76-not reached) and the 5-year OS rate of the entire cohort was 70.3% (95% CI: 57.1-80.2). In the entire cohort, patients with IKZF1 del had a strong trend of poor OS compared to IKZF1 WT patients (IKZF1 del vs. WT, median survival: 76 months vs. not reached, 5-year OS: 57.8% vs. 81.2%, P = 0.082), while PAX5 and CDKN2A deletion status did not affect OS. Among the dasatinib-treated subgroup, IKZF1, PAX5, and CDKN2A del status did not affect OS. Among the patients with IKZF1 del, ponatinib-treated subgroup had better prognosis than dasatinib-treated subgroup (dasatinib vs. ponatinib, median survival: not reached vs. 80 months, 5-year OS: 40.0% vs. 77.2%, P = 0.0337) [Table 2]. In ponatinib-treated subgroup, CDKN2A del was associated with significantly worse OS (CDKN2A del vs. WT, median survival: both not reached, 5-year OS: 66.7% vs. 96.0%, P = 0.0259), while IKZF1 del was associated with a trend of worse OS (IKZF1 del vs. WT, median survival: both not reached, 5-year OS: 77.0% vs. 95.0%, P = 0.161). One of limitations of this study is the number of cases is small.
Discussion
In the patients with Ph+ B-ALL treated with Hyper-CVAD/MA plus dasatinib or ponatinib, IKZF1 del did not affect CMR at 3 months while it was associated with a trend toward worse OS. In the patients harboring IKZF1 del, ponatinib-treated subgroup had better prognosis than dasatinib-treated subgroup. Further investigation is needed to determine the prognostic significance of IKZF1 del in individual treatment subgroup.
Short:AstraZeneca: Consultancy; Amgen: Honoraria; Takeda Oncology: Consultancy, Research Funding. Kantarjian:Novartis: Research Funding; Pfizer: Honoraria, Research Funding; Jazz Pharma: Research Funding; AbbVie: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Amgen: Honoraria, Research Funding; Cyclacel: Research Funding; BMS: Research Funding; Astex: Research Funding; Takeda: Honoraria; Ariad: Research Funding; Immunogen: Research Funding; Agios: Honoraria, Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees. Jain:Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding. Sasaki:Pfizer: Consultancy; Otsuka: Honoraria. Ravandi:Xencor: Consultancy, Research Funding; Macrogenix: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Selvita: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Menarini Ricerche: Research Funding. Konopleva:Genentech: Honoraria, Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Calithera: Research Funding; Kisoji: Consultancy, Honoraria; Ascentage: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding; Cellectis: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Eli Lilly: Research Funding; Forty-Seven: Consultancy, Honoraria; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Ablynx: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties. Garcia-Manero:Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding; Helsinn: Research Funding; Amphivena: Consultancy, Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding. Takahashi:Symbio Pharmaceuticals: Consultancy. Jabbour:Cyclacel LTD: Research Funding; Pfizer: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal