Background
Chimeric Antigen Receptor (CAR)-T cell therapy has been successfully clinically deployed in the context of B-cell malignancies, paving the way for further development also in Acute Myeloid Leukemia (AML), a still unmet clinical need in the field of oncohematology. Among the potential AML targetable antigens, CD33 is so far one of the main validated molecule.
Objectives
The aim of the present study was to optimize a non-viral gene transfer method to engineer Cytokine-Induced Killer (CIK) cells with a CD33.CAR by using a novel version of the Sleeping Beauty (SB) transposon system, named "SB100X-pT4". Further, a preclinical assessment of SB-modified CD33.CAR-CIK cells was performed in chemoresistant AML Patient-Derived Xenografts (PDX), in order to address the unmet need of targeting drug-resistant AML cells.
Methods
Donor derived-CIK cells were stably transduced with a CD33.CAR by exploiting the novel hyperactive SB100X transposase and the pT4 transposon (SB100X-pT4). The novel SB system has been in vitro compared to the previous established SB11-pT. In vitro anti-AML activity of CD33.CAR-CIK cells was assessed by flow cytometry-based cytotoxicity (AnnV-7AAD), proliferation (CFSE) and cytokine production (intracellular IFNg and IL2 detection) assays. In vivo efficacy was evaluated in NSG mice transplanted with MA9-NRas AML cell line or PDX samples. A xenograft chemotherapy model mimicking induction therapy ("5+3" Ara-C and doxorubicin) was exploited to examine the potential benefit of CD33.CAR-CIK cells on chemoresistant/residual AML cells.
Results
By significantly reducing the amount of DNA transposase, the novel SB100X-pT4 combination resulted in higher CAR levels than the SB11-pT. SB100X-pT4-modified CD33.CAR CIK cells showed efficient expansion after 3 weeks (median fold increase of 38.89, n=4). Both transpositions conferred to CD33.CAR-CIK cells a specific killing (up to 70%) against CD33+ AML target cell lines and primary AML cells. The anti-AML proliferative response of SB-modified CD33.CAR-CIK cells was also considerable (up to 70% of CFSE diluted CAR-CIK cells), as well as the cytokine production (up to 35% for IFN-γ and up to 25% for IL-2).
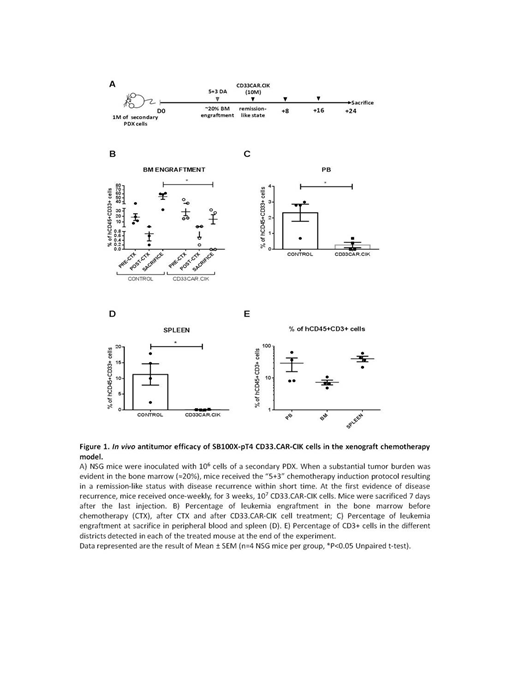
To evaluate the effect of SB100X-pT4-modified CD33.CAR-CIK cells particularly on Leukemia Initiating Cells (LICs), CD33.CAR-CIK cells were administered as an "early treatment" in mice transplanted with the MA9-NRas cell line, which retains a high frequency of LICs. At sacrifice, CD33.CAR-CIK cell-treated mice showed a significant bone marrow (BM) engraftment reduction (median 27.80 for the untreated group and 22.60 for the unmanipulated CIK cells vs 6.45 for CD33.CAR-CIK cell, n=4 NSG mice per group, p= 0.02). PDX of two different AML samples at the onset were established to be used as models mimicking different disease conditions. In an "early treatment" model using secondary transplanted PDX, a setting which presumably reflects the typical LIC properties, a clear engraftment reduction in the treated cohort was observed, nearly undetectable in 2/5 mice, as compared to the untreated mice (up to 70% in BM). A significant leukemia reduction was also measured in the peripheral blood and spleen of treated mice, showing CD33.CAR-CIK cell potential of reducing AML dissemination in the periphery. When ex vivo re-exposed to CD33.CAR-CIK cells residual AML cells were still sensitive to the treatment, indicating that no resistance mechanisms occurred. CD33.CAR-CIK cells were also effective in a second model by which the treatment started when AML engraftment was clearly manifested in the BM (> 1%). Finally, when starting CD33.CAR-CIK cell treatment after disease recurrence post induction therapy, a significant disease reduction was observed in the CD33.CAR-CIK-treated group, reaching undetectable levels in half of the mice, as compared to chemotherapy-only treated mice (up to 60% of engraftment in BM)(Figure 1).
Conclusions
The employment of a non-viral SB-based CD33.CAR-gene transfer approach, which is overall associated to less cumbersome protocols and reduces the cost of goods, offers a unique alternative to current viral-based strategies to be explored in the setting of resistant forms of AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal