Invasive fungal infections (IFIs) remain a major clinical burden due to their morbidity and mortality, particularly in patients with acute leukemias and allogeneic HSCT which represent the main risk factors for proven/probable IFI in hematology. We conducted a study in France which enrolled 677 patients with acute myeloid leukemia (AML) receiving intensive chemotherapy from 34 ALFA centers. This study confirmed the significant lower rate of proven/probable IFI in patients who received antifungal prophylaxis (AFP), and that IFI was associated with an increased early mortality rate.
The trial recommended laminar air flow rooms and posaconazole AFP according to the 2009 recommendations of the European Conference on Infections in Leukaemia (ECIL). IFI were graded as proven/probable or possible by local investigators. Two central review processes were performed. All study data were centrally reviewed by hematological expert according to the EORTC classification. In parallel, available CT-scans were reviewed by two independent experts (hematologist and pneumologist).
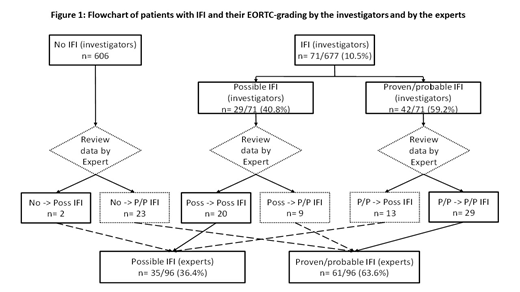
We showed three supplementary important observations: (1) Despite the ECIL recommendations, 30% of patients (203/677) did not receive any AFP, and 91 patients (13%) received another antifungal agent than the one recommended. (2) with regards to the IFI grading (Figure 1), 71 IFI were diagnosed and graded by the investigators. After review by the experts, the grade was maintained for 49/71 IFI [69%, 20 possible and 28 proven/probable IA and 1 proven/probable invasive candidiasis (IC)], while 9 possible IFI (13%) (8 IA and 1 IC) were upgraded as proven/probable, and 13 proven/probable IFI (18%) (13 IA) were downgraded as possible. Twenty-five IFI were not graded by the investigators including 3 cases of IA graded by the experts (2 proven/probable and 1 possible) for whom antifungal prophylaxis was pursued, and 22 cases of other IFI graded only by the experts: 15 IC and 7 invasive mucormycosis (IM) all proven/probable, for whom AFP was modified for a curative therapy. In addition, chest imaging data of 37 patients were centrally reviewed, and 21 (57%) were reclassified. The review of imaging data was 100% consistent with the EORTC-based expert review. The experts graded more proven/probable IFI than the investigators with 9.0% (61/677) versus 6.2% (42/677). (3) Among patients without IFI, the rate of complete hematological remission was higher (513/581, 88.3%) versus those with IFI (77/96, 80.2%) (p=0.04). Among patients with IFI, the rate of posaconazole-based AFP was 45.5% (35/77) for those who achieved CR, vs. 63.2% (12/19) for those who did not achieve CR.
In conclusion, we showed in this very high-risk population, ECIL recommendations were followed only in 57% of patients. The frequent "misgrading" of the IFI (33% of IA up or downgraded and 92% of other IFI) has an impact on their appropriate management. Another important message is that haematological failure is associated with more IFI despite the AFP.
De Botton:Pierre Fabre: Consultancy; AbbVie: Consultancy; Forma: Consultancy, Research Funding; Daiichi: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Agios: Consultancy, Research Funding; Servier: Consultancy; Bayer: Consultancy; Pfizer: Consultancy; Celgene Corporation: Consultancy, Speakers Bureau; Syros: Consultancy; Astellas: Consultancy. Bertoli:Astellas: Consultancy, Honoraria; Jazz Pharmaceuticals: Honoraria; Sanofi: Honoraria; Daiichi Sankyo: Consultancy. Castaigne:Pfizer: Consultancy. Vey:Janssen: Honoraria; Novartis: Consultancy, Honoraria. Dombret:CELGENE: Consultancy, Honoraria; AGIOS: Honoraria; Institut de Recherches Internationales Servier (IRIS): Research Funding. Thomas:DAICHI: Honoraria; ABBVIE: Honoraria; PFIZER: Honoraria; INCYTE: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal