Background: Sickle cell disease (SCD) is an inherited disorder in which pathology is driven by hemoglobin (Hb) polymerization and red blood cell sickling, leading to chronic anemia and hemolysis as well as episodic vaso-occlusive crises (VOC). These manifestations of SCD contribute to the cumulative organ damage that leads to disability, reduced quality of life, and accelerated mortality. In particular, VOCs and their associated episodic pain are a hallmark symptom of SCD and frequently require emergency medical attention.
Voxelotor is a first-in-class sickle hemoglobin-polymerization inhibitor in development for the treatment of SCD. It has demonstrated robust, rapid, and sustained improvements in patient Hb levels with numerically fewer VOCs compared with placebo, which suggests that viscosity was not increased with voxelotor treatment. The objective of this study was to further explore this observation by examining the association between absolute Hb achieved by voxelotor treatment and VOC incidence rate. In addition, to inform on the potential for symptom exacerbation after drug discontinuation, rates of VOCs after voxelotor discontinuation were analyzed.
Methods: The HOPE trial is a phase 3, randomized, placebo-controlled, double-blind, multicenter study comparing the efficacy and safety of voxelotor (1500 mg and 900 mg daily) versus placebo for ≥24 weeks in patients with SCD aged 12 to 65 years. The primary endpoint is the percentage of patients with a Hb response at week 24, defined as a >1.0 g/dL increase in Hb. Secondary endpoints included the annualized incidence rate of VOC. This abstract reports a post hoc analysis of VOC incidence in the per-protocol population stratified by Hb level at 24 weeks of treatment. In addition, VOCs in patients who discontinued voxelotor and completed a 28-day follow-up are reported here (data cutoff October 31, 2018).
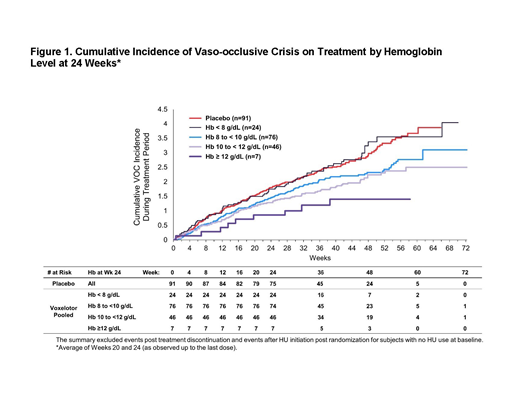
Results: The proportion of patients with ≥1 VOC was 67.0% (59/88) in the voxelotor 1500 mg group, 66.3% (61/92) in the voxelotor 900 mg group, and 69.2% (63/91) in the placebo group. Overall, the annualized adjusted incidence rate of VOCs (the number of crises per person-year) was 2.77 in the voxelotor 1500 mg group, 2.76 in the voxelotor 900 mg group, and 3.19 in the placebo group. When stratified by Hb level after 24 weeks of treatment, the incidence of VOCs was generally lower in patients who achieved higher absolute Hb levels on voxelotor treatment compared with placebo (Figure 1).
Patients who discontinued voxelotor were also observed for 28 days post-treatment. At the time of data cutoff, 55 patients (n=21, voxelotor 1500 mg; n=17, voxelotor 900 mg; n=17, placebo) had discontinued treatment and had post-treatment follow-up. During the 28-day period after treatment discontinuation, 5 patients in the voxelotor 1500 mg group reported 6 VOCs; 3 patients in the voxelotor 900 mg group reported 3 VOCs; and 5 patients in the placebo group reported 8 VOCs. The estimated incidence rates of post-treatment VOCs were 4.63, 4.30, and 7.01 in the voxelotor 1500 mg, voxelotor 900 mg, and placebo groups, respectively.
Conclusions: Patients who achieved the greatest absolute Hb level after 24 weeks of treatment with voxelotor had numerically fewer VOCs, suggesting that increasing Hb levels resulting from voxelotor treatment did not lead to a viscosity-related increase in risk of vaso-occlusion. Following drug discontinuation, there was a numerically lower incidence of VOCs in the voxelotor arms compared with placebo. Altogether, these results suggest that voxelotor treatment safely raises Hb without causing a viscosity-related increased risk of VOC and that treatment discontinuation did not increase risk for VOC.
Vichinsky:GBT: Consultancy, Research Funding; bluebird bio: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Telfer:ApoPharma: Membership on an entity's Board of Directors or advisory committees, Other: Speaker activities, clinical trial activities; Terumo: Honoraria, Other: Speaker activity; Pfizer: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: clinical trial activity; Kyowa Kirin Limited: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: clinical trial activities; Napp Pharma: Other: clinical trial involvement; Celgene: Other: clinical trial involvement; Bluebird Bio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Inati:Global Blood Therapeutics: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novonordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. Tonda:Global Blood Therapeutics: Employment, Equity Ownership. Tong:Global Blood Therapeutics: Employment, Equity Ownership. Agodoa:Global Blood Therapeutics: Employment, Equity Ownership. Lehrer-Graiwer:Global Blood Therapeutics: Employment, Equity Ownership. Ataga:Modus Therapeutics: Honoraria; Emmaus Life Sciences: Honoraria, Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Bioverativ: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal