Kidney disease is a common complication in sickle cell anemia (SCA), which leads to increased morbidity and early mortality. The National Kidney Foundation guidelines use an estimated glomerular filtration rate (eGFR) cutoff of 60 mL/min/1.73m2 to define chronic kidney disease (CKD). However, many SCA patients have an elevated baseline eGFR due to low serum creatinine levels from reduced muscle mass, abnormal tubular secretion of serum creatinine into the urine, and/or high cardiac output from the hemolytic anemia (PMID: 23894560, 20185605). The standard definition of CKD may represent a greater decline from "normal" kidney function in SCA patients compared to the general population.
In two independent SCA (Hb SS or Sβ0-thalassemia) cohorts, we investigated eGFR cutoffs for when kidney dysfunction, assessed by altered electrolyte (serum potassium) and acid-base (serum HCO3) balance, osteodystrophy (alkaline phosphatase), increased blood pressure and impaired erythropoiesis (hemoglobin < 9 g/dL and absolute reticulocyte count < 250 x 109/L), were observed. Laboratory and clinical variables were obtained at outpatient visits at the time of enrolment. The eGFR categories were grouped as follows: > 120, 90 - 120, 60 - 89, and < 60 mL/min/1.73m2. We compared linear and categorical variables by eGFR category using the test for linear trend and Cochran's test for linear trend, respectively. Mean values and standard error bars are provided in the figures.
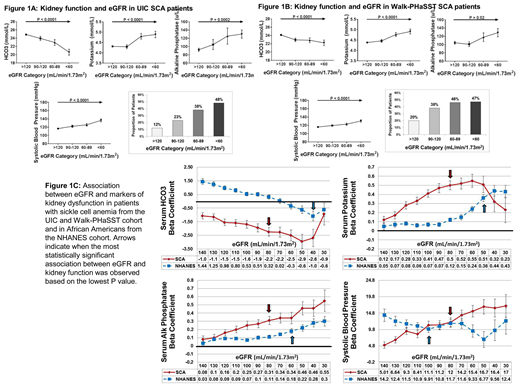
We first conducted our analysis in 270 SCA patients treated at the University of Illinois at Chicago (UIC). The median age of the cohort was 31 years (interquartile range (IQR), 23 - 42 years), 59% were female, and 52% were on hydroxyurea. The proportion of SCA patients by eGFR category was as follows: 69% with eGFR > 120 mL/min/1.73m2, 13% with eGFR 90 - 120 mL/min/1.73m2, 9% with eGFR 60 - 89 mL/min/1.73m2, and 9% with eGFR < 60 mL/min/1.73m2. With progressively lower eGFR category, we observed higher serum potassium, alkaline phosphatase, systolic blood pressure, and proportion of patients with ineffective erythropoiesis and lower serum HCO3 (Figure 1A) (P ≤ 0.0002).
We repeated our analyses in 456 SCA patients from the multi-center Walk-Treatment of Pulmonary Hypertension and Sickle cell disease with Sildenafil Therapy (Walk-PHaSST) cohort. The median age of the cohort was 34 years (IQR, 24 - 45 years), 52% were female, and 43% were on hydroxyurea. The proportion of SCA patients by eGFR category was as follows: 68.5% with eGFR > 120 mL/min/1.73m2, 15% with eGFR 90 - 120 mL/min/1.73m2, 8.5% with eGFR 60 - 89 mL/min/1.73m2, and 8% with eGFR < 60 mL/min/1.73m2. Manifestations of reduced kidney function were progressively worse with lower eGFR category (Figure 1B) (P ≤ 0.02).
We then assessed the association of eGFR with altered kidney function using the test for linear trend in a combined analysis of SCA patients from UIC and Walk-PHaSST as well as in non-SCA African Americans adults from the National Health and Nutrition Examination Survey (NHANES) cohort (n = 1331). The median age of the NHANES cohort was 48 years (IQR, 29 - 62) and 53% were female. The associations between eGFR and kidney dysfunction, based on the beta coefficients, were stronger for serum HCO3, potassium, and alkaline phosphatase in SCA versus non-SCA patients (Figure 1C). The most significant associations between eGFR and kidney dysfunction were observed at an eGFR cutoff of 80 mL/min/1.73m2 for SCA patients, which was higher than the cutoffs observed in non-SCA patients for HCO3 (40 mL/min/1.73m2), potassium (50 mL/min/1.73m2), and alkaline phosphatase (60 mL/min/1.73m2).
In conclusion, we demonstrate that kidney dysfunction occurs in SCA patients at eGFR values that are above the standard thresholds currently used to define CKD. Manifestations of kidney dysfunction progressively worsen with lower eGFR category and the differences are most significant at an eGFR < 80 mL/min/1.73m2. Future studies to redefine kidney disease in SCA based on eGFR may help identify high-risk patients for earlier intervention strategies and for the avoidance of potential nephrotoxins, such as nonsteroidal anti-inflammatory drugs and intravenous contrast.
Saraf:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding. Derebail:Retrophin: Consultancy; RTI: Honoraria; Novartis: Consultancy. Gladwin:Globin Solutions, Inc: Patents & Royalties: Provisional patents for the use of recombinant neuroglobin and heme-based molecules as antidotes for CO poisoning; United Therapeutics: Patents & Royalties: Co-inventor on an NIH government patent for the use of nitrite salts in cardiovascular diseases ; Bayer Pharmaceuticals: Other: Co-investigator. Gordeuk:Global Blood Therapeutics: Consultancy, Honoraria, Research Funding; Emmaus: Consultancy, Honoraria; CSL Behring: Consultancy, Honoraria, Research Funding; Inctye: Research Funding; Modus Therapeutics: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Research Funding; Ironwood: Research Funding; Imara: Research Funding. Little:Hemex Health, Inc.: Patents & Royalties; GBT: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal