Patients on hydroxyurea who achieve maximum tolerated dose (MTD), defined by a target level of mild myelosuppression, may have greater laboratory and clinical benefits than those maintained on a lower dose. MTD is currently determined by gradual dose escalation, a process that often takes six to twelve months. Using data from a previous cohort of pediatric patients escalated to MTD on hydroxyurea, we have developed an equation incorporating baseline serum creatinine, body mass index, and absolute reticulocyte count to predict individualized MTD for patients initiating therapy. The NDEPTH (Novel Dose Escalation to Predict Treatment with Hydroxyurea) study is a prospective, open-label, randomized controlled trial consisting of two treatment arms: a standard arm utilizing a current published dose-escalation protocol for achieving hydroxyurea MTD; and an alternative treatment arm utilizing the dose-prediction equation to determine MTD prior to initiation of treatment. The primary endpoint of the study is time to MTD for each arm. Additional endpoints include analysis of safety and clinical and laboratory response to hydroxyurea therapy
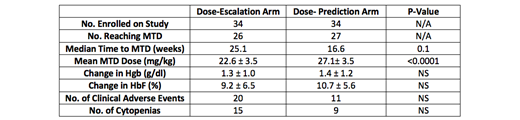
We recruited 70 pediatric patients to the study, 68 of whom were randomized equally to the standard and dose-prediction arms of the study. There were no significant differences in baseline characteristics between study participants in the two arms. Twenty-six study participants in the standard arm and 27 in the dose-prediction arm successfully reached MTD. Mean MTD was significantly higher in the dose-prediction arm than the standard arm (27.1 mg/kg vs. 22.6 mg/kg; P <0.0001). The dose-prediction equation calculated the actual MTD for all study participants reaching MTD with a high degree of accuracy (r = 0.43; p = 0.001). On an intention-to-treat log-rank analysis of time to MTD, median time to MTD or censoring on the standard arm was 25.1 weeks while that on the dose-prediction arm was 16.6 weeks (p = 0.1), revealing a trend towards quicker achievement of MTD in the dose-prediction arm. There were 20 episodes of cytopenias on the standard arm and 11 on the dose-prediction arm during the study period, none of which were clinically significant. The number of clinical adverse events over 12 months were also similar between the two study arms (15 in the standard arm and 9 in the dose-prediction arm).
In summary, the dose-prediction equation determined actual MTD with a high degree of accuracy and resulted in a significantly higher final hydroxyurea dose for study participants in the dose-prediction arm than that achieved in the standard arm, indicating that young children may be able to tolerate higher hydroxyurea doses than can be achieved by standard dose escalation. The incidence of adverse clinical and laboratory events was similar between the two study arms. Based on these results, we conclude that our dose-prediction method of determining hydroxyurea MTD can be used to safely and rapidly achieve MTD, obviating the delayed MTD and the requirement for frequent clinical and laboratory monitoring associated with standard dose escalation.
George:Global Blood Therapeutics; Pfizer: Consultancy, Honoraria. Fasipe:Novartis: Consultancy, Honoraria; Pfizer and American College of Emergency Physicians: Research Funding. Ware:Bristol Myers Squibb: Other: Research Drug Donation; Addmedica: Other: Research Drug Donation; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: DSMB; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Nova Laboratories: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal