Incident stroke, both primary and recurrent are common in children with sickle cell anemia (SCA) in Jamaica with incidences of 7.8% by 14 years of age and 29/100 person-years respectively. Cerebral vasculopathy manifested by elevated transcranial doppler (TCD) velocity (>170 cm/sec) is the major risk factor for both with a prevalence of 19.8% among Jamaican children with SCA. Chronic blood transfusion is the standard of care to reduce stroke risk and recurrence in children with SCA in high income countries, but in low-resource settings where blood availability, safety and local acceptance are limited, hydroxyurea (HU) is emerging as a viable alternative. However, the efficacy of HU for incident stroke prevention in children with newly diagnosed severe cerebrovascular disease without the use of transfusions in a low resource setting like Jamaica is unclear. The EXpanding Treatment for Existing Neurological Disease (EXTEND) trial (ClinicalTrials.gov NCT02556099) was designed to investigate the effects of open label HU on TCD velocities after 18 months of treatment, compared to the pre-treatment value. Secondary aims included the effects of HU on the incidence of neurological events including magnetic resonance imaging (MRI), magnetic resonance angiography (MRA) changes, non-neurological events, hematological responses and toxicity.
We enrolled 43 children with SCA, 2 to 17 years of age , between Nov 2014 and April 2016, stratified into 3 groups: Low Risk Group (LRG) - On Hydroxyurea with TCD ≥170 cm/sec, N=12; Medium Risk Group (MRG) - HU naive with TCD ≥170 cm/sec, N=21; and High Risk Group (HRG) - Previous Stroke, N=10. All children received HU initially at 20 mg/kg/day followed by two monthly dose escalation using weight and pre-defined laboratory criteria to maximum tolerated dose (MTD) and thereafter 3 monthly visits. The average HU dose at MTD (mean±1SD) was 25.3±0.4 mg/kg. TCD was performed every 6 months according to the Stroke Prevention in Sickle Cell Anemia (STOP) protocol.
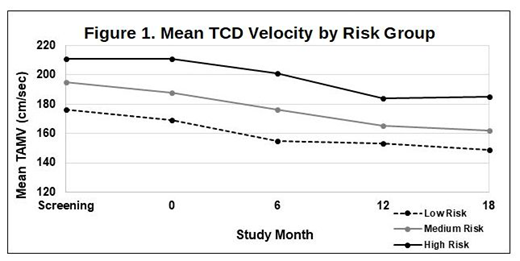
The average age at enrollment was 7.7±2.6 years, with MRG significantly younger than HRG (6.7±2.0 years vs 9.2±3.7 years; p<0.012). At baseline, there were no significant differences in gender or previous history of vaso-occlusive painful events, dactylitis, Acute Chest Syndrome, Acute Splenic Sequestration, transfusion history, or frequency of hospitalization among the groups. As expected, the LRG cohort on HU had a higher baseline hemoglobin concentration (Hb=9.1±1.3 g/dl) than MRG (7.8±0.8 g/dl) and HRG (7.4±0.9 g/dl), p<0.003) but there were no significant differences in % fetal hemoglobin (%HbF, cohort average = 13.5±6.8%) or white blood cell count (WBC, cohort average = 11.9±3.7 x 109/L). Mean TCD velocity was lowest in LRG and highest in HRG (Fig 1). The primary study endpoint was change in TCD velocity after 18 months of HU treatment, and across all groups TCD velocities decreased by an average of 24±30 cm/sec, with no differences by group (Fig 1). The entire cohort was observed for 697.2 person-months on protocol HU therapy with an incidence rate for new infarcts (IR) of 12/100 person-years. HRG had significantly higher (p<0.004) IR with 6 events in 5 participants (IR=43/100 person-years) compared with 1 new infarct in the LRG (IR=6/100 person-years) and, none in MRG (IR=0). All children with new infarcts during the study had abnormal baseline MRI and MRA, and some had previous stroke recurrence.
Laboratory benefits of HU included clinically significant increases in Hb (2.0±0.9 g/dl) and (1.7±0.7 g/dl) in MRG and HRG as well as increases in %HbF, (17.8±10.7 ) in MRG and (14.4±14) in HRG plus significant decreases in WBC (6.5±3.7 x 109/L) and (4.5±3.3 x 109/L) in MRG and HRG respectively. There were no significant changes in hematology for LRG. Overall, HU was well-tolerated with 0.53 recorded toxicities/year during 697.2 patient-months of treatment.
Treatment with HU at MTD for 18 months in children at high risk for strokes was effective in lowering TCD velocities with laboratory benefits and was safe. However, while the overall incidence of new neurological events was low in children with elevated TCD velocities, children with previous stroke experienced higher rates of stroke recurrence on HU therapy. Thus, the incidence of neurological events in children with previous stroke may be related to severe vasculopathy that is challenging to manage using HU, transfusions, or newer disease-modifying therapies.
Rankine-Mullings:Nova Laboratories Limited, Martin House, Gloucester Crescent, Wigston, Leicester, LE18 4YL: Other: I am Principal investigator for the performance site at the University of the West Indies for the protocol A prospective open label, pharmacokinetic study of an oral Hydroxyurea solution in children with sickle cell anemia . Knight-Madden:Global Blood Therapeutics: Research Funding; Global Blood Therapeutics: Other: Sponsor of a conference held by Sickle Cell Unit in 2017; Addmedica: Other: Sponsor of a conference held by Sickle Cell Unit in 2017; BlueBird Bio: Other: Sponsor of a conference held by Sickle Cell Unit in 2017; Nova Laboratories: Other: Sponsor of a conference held by Sickle Cell Unit in 2017; Abbot International: Other: Sponsor of a conference held by Sickle Cell Unit in 2017; Pfizer: Other: Advisory Board on SCD 2017; Abbott Nutrition: Other: Sponsor of a conference held by Sickle Cell Unit in 2017; Nova Laboratories: Advisory Board on SCD 2017, Research Funding. Adams:Bluebird: Consultancy; GBT: Consultancy, Other: consultancy to companies GBT and Blueburd Bio. Ware:CSL Behring: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Other: Research Drug Donation; Addmedica: Other: Research Drug Donation; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: DSMB; Nova Laboratories: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal