Background: While immunotherapy withCD19 specific CAR T cells for relapsed/refractory (R/R) B-ALL achieves MRD negative remission in nearly all patients, relapse occurs in approximately half of patients and is frequently associated with early loss of CAR T cell persistence. Low CD19 antigen burden in the bone marrow prior to lymphodepletion and rapid contraction of CAR T cells in the blood after engraftment are predictive of early loss of CAR T cell persistence. We hypothesize that episodic antigen exposure using CD19t T cell antigen presenting cells (T-APCs) can trigger CD19 CAR T cell proliferation and re-activation in vivo, resulting in more durable CAR T cell persistence and diminished risk of CD19+ relapse. Here we report our experience to date using T-APCs following CD19 CAR T cell therapy for children and young adults with R/R B-ALL on clinical trial PLAT-03 (NCT03186118).
Methods: Patient-derived T-APC products were manufactured from cryopreserved CD4/CD8 selected T cells. Cells were then activated with anti-CD3/CD28 beads, transduced with a lentiviral vector to express a truncated human CD19 (CD19t), and expanded in culture for 10 days. Subjects <25kg received 10x106 T-APCs/kg/dose and those ≥25kg received a flat dose of 5x108 T-APCs/dose, for up to a total of six-monthly doses of T-APCs. Adverse events were graded according to CTCAEv4.
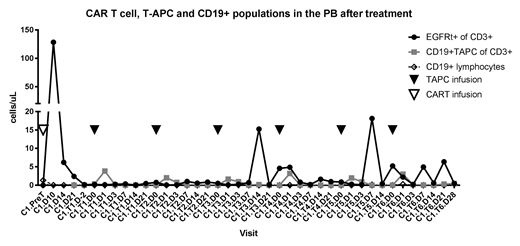
Results: Fourteen subjects (8-26 yrs) have been enrolled; 8 with low CD19 antigen burden, 5 with rapid CAR T cell contraction, and 1 subject with early loss of CAR T cell persistence on a predecessor study who received a re-infusion of CD19 CAR T cells on this study followed by T-APCs. T-APCs were successfully manufactured in 14/14 subjects. Two subjects lost CAR T cell persistence prior to T-APC infusion and were ineligible to receive T-APCs. To date, 11 subjects have received at least one dose of T-APCs. One of 11 subjects experienced a grade 3 febrile infusion reaction within hours of the 2nd dose of T-APCs, prohibiting further dosing. There were no other related adverse events (AEs) > grade 2 among the 11 subjects, and no cytokine release syndrome or neurotoxicity has been observed. An increase in detectable CD19 CAR T cells occurred in all subjects following T-APCs, and T-APCs can be transiently detected following infusion (Figure 1). Of the 10 treated subjects with low CD19 antigen burden or rapid T cell contraction, 8/10 had CAR T cell persistence beyond Day 63, as evidenced by B cell aplasia. At last follow-up, 5/10 have ongoing B cell aplasia, with a median follow up of 8.8 months (range, 2-18.5 months). The estimated 1-year leukemia free survival (LFS) is 69.2%.
Conclusion: This first-in-human study of CD19t T-APCs demonstrates the ability to successfully manufacture T-APCs from stored apheresis products collected for CAR T cell production. In 11 subjects receiving at least one T-APC dose to date, there has been one T-APC infusion reaction and no other significant associated toxicity. Early evidence of efficacy demonstrated by secondary expansion of CAR T cells suggests the potential of CD19t T-APCs to enhance durable CD19 CAR T cell persistence.
Gardner:Novartis: Honoraria. Jensen:Bluebird Bio: Research Funding; Juno Therapeutics, a Celgene Company: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal