Background: NGS of myeloid mutations is an integral part of AML clinical decision-making. There is currently no information regarding concordance between NGS panels in AML using samples from the same patient across various platforms in different diagnostic laboratories. To study this important question, we analyzed NGS of myeloid mutations in diagnostic samples from The Beat AML Master Trial (BAMT) for newly diagnosed older AML patients, and compared variant calls made between institutional laboratories enrolling the study subject with those made by Foundation Medicine (FM), the central laboratory used for treatment assignment in this precision medicine trial.
Methods: We identified newly diagnosed AML patient samples (peripheral blood (PB) and/or bone marrow (BM)) from 2 lead institutions in the BAMT(Ohio State, OSU and Oregon Health and Sciences University, OHSU) that were analyzed by both the institutional and by FM from Nov 15, 2016 to Apr 15, 2019. Samples sent to both laboratories >3 days apart were excluded. Samples were analyzed at the institutional laboratories using their respective NGS mutational panels and by FM using the FoundationOne®Heme(FMH) NGS panel which utilizes capture based sequencing. The OSU NGS assay utilizes sequencing on Illumina MiSeq. The OHSU NGS assay employs semiconductor-based sequencing (Ion Torrent PGM platform). The variant allele frequency (VAF) sensitivity for detection for all 3 laboratories range from 1-2%. We evaluated the ability to identify mutations in 8 genes : FLT3, IDH1/ 2, NPM1, TET2, DNMT3A, WT1 and TP53 used in treatment assignment in theBAMT. A detection cutoff of 2% was used to define the presence or absence of a mutation. Overall, agreement was defined as the number of times the local and central laboratories made the same call divided by the total number of patients. Sensitivity was defined as the number of present calls made locally divided by the number of present calls made centrally, and specificity as the number of absent calls made locally divided by the number of absent calls made centrally. The overall kappa statistic, controlling for institution, provided another measure of agreement between local and central calls, where a value of 1 indicates perfect agreement.
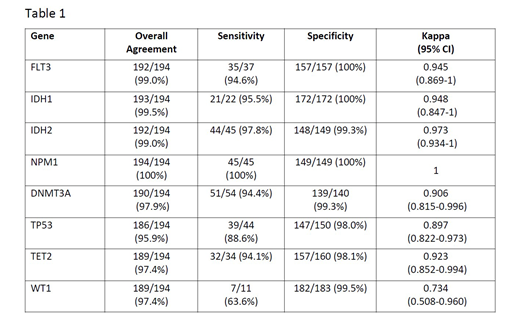
Results: 194 patient samples were identified using methods above and analyzed locally at the screening institution (125 at OSU, 69 at OHSU) and centrally at FM. Type of tissue analyzed for variants between local site and FM were 59 PB, 129 BM, and 6 with BM/PB mismatch. Overall agreement in presence/absence calls between local and central results for each of the 8 genes was over 95% (Table 1). There was perfect agreement for NPM1. The sensitivity was above 94% for all genes except TP53 (88.6%) and WT1 (63.6%). Failure to detect a mutation locally was primarily due to reporting of all TP53 variants, including variants of unknown significance (VUS) (5) by FM as agreed upon in the study protocol, detection at low levels below local site sensitivity cutoff (1), detection of variants in a portion of gene not covered at the local site(1)and possible artifact (1). For the WT1 gene, discordance in 5 samples included VUS (3) reported by FM ,a variant detected in a portion of the gene not covered at the local site(1).and difference in leukemic tissue analyzed with mutation not detected by the central laboratory on a PB sample, and present at the institutional lab on a BM sample; affecting the overall agreement and specificity but not sensitivity. Specificity was at least 98% for each of the 8 genes. Finally, most discrepancies in reported mutations in FLT3 (n=2), IDH1 (n=1), IDH2 (n=2), DNMT3A (n=4) and TET2 (n=5) were due to reporting of VUS in one laboratory and not by another.
Conclusion: Detection of pathogenic myeloid mutations using orthogonal assays showed a high degree of concordance for genes used in therapeutic assignment on the BAMT.The small number of discordant results, in TP53 and WT1, were attributed to the reporting of VUS. This study illustrates the importance of quality control and standardization as NGS continues to be widely utilized in AML for clinical decision making, with a variety of platforms across multiple laboratories. Our next steps involve evaluating the differences in VAFs reported between local and central laboratories when a given mutation is identified, as well as the potential reasons for observed differences and clinical implications of known pathogenic mutations vs putative VUS.
Borate:Daiichi Sankyo: Consultancy; AbbVie: Consultancy; Novartis: Consultancy; Takeda: Consultancy; Pfizer: Consultancy. Vergilio:Foundation Medicine: Employment; Roche Holding AG: Equity Ownership. Stein:Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo, Inc.: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma US, Inc: Membership on an entity's Board of Directors or advisory committees; Bioline: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; PTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees. Patel:France Foundation: Honoraria; Dava Oncology: Honoraria; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Baer:Astellas: Research Funding; Abbvie: Research Funding; AI Therapeutics: Research Funding; Forma: Research Funding; Incyte: Research Funding; Kite: Research Funding; Takeda: Research Funding. Stock:Daiichi: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Research to Practice: Honoraria; UpToDate: Honoraria; Kite, a Gilead Company: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Schiller:Amgen: Other, Research Funding; Astellas: Research Funding; Biomed Valley Discoveries: Research Funding; Bristol Myer Squibb: Research Funding; Celgene: Research Funding, Speakers Bureau; Constellation Pharmaceutical: Research Funding; Daiichi Sankyo: Research Funding; Eli Lilly and Company: Research Funding; FujiFilm: Research Funding; Genzyme: Research Funding; Gilead: Research Funding; Incyte: Research Funding; J&J: Research Funding; Jazz Pharmaceuticals: Honoraria, Research Funding; Karyopharm: Research Funding; Novartis: Research Funding; Onconova: Research Funding; Pfizer Pharmaceuticals: Equity Ownership, Research Funding; Sangamo Therapeutics: Research Funding; Agios: Research Funding, Speakers Bureau. Blum:AmerisourceBergen: Consultancy; Boehringer Ingelheim: Research Funding; Celgene: Research Funding; Astellas,: Research Funding; Xencor: Research Funding; Forma: Research Funding. Kovacsovics:Pfizer: Research Funding; Jazz: Consultancy; Novartis: Research Funding; Abbvie: Research Funding; Amgen: Consultancy, Research Funding. Foran:Agios: Honoraria, Research Funding. Druker:Pfizer: Research Funding; OHSU (licensing fees): Patents & Royalties: #2573, Constructs and cell lines harboring various mutations in TNK2 and PTPN11, licensing fees ; Cepheid: Consultancy, Honoraria; Aileron Therapeutics: #2573, Constructs and cell lines harboring various mutations in TNK2 and PTPN11, licensing fees , Membership on an entity's Board of Directors or advisory committees; ALLCRON: Membership on an entity's Board of Directors or advisory committees; Amgen: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Aptose Biosciences: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Beta Cat: Membership on an entity's Board of Directors or advisory committees, Other: Stock options; GRAIL: Equity Ownership, Other: former member of Scientific Advisory Board; Patient True Talk: Consultancy; The RUNX1 Research Program: Membership on an entity's Board of Directors or advisory committees; Vivid Biosciences: Membership on an entity's Board of Directors or advisory committees, Other: Stock options; Beat AML LLC: Other: Service on joint steering committee; CureOne: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Gilead Sciences: Other: former member of Scientific Advisory Board; ICON: Other: Scientific Founder of Molecular MD, which was acquired by ICON in Feb. 2019; Monojul: Other: former consultant; Novartis: Other: PI or co-investigator on clinical trial(s) funded via contract with OHSU., Patents & Royalties: Patent 6958335, Treatment of Gastrointestinal Stromal Tumors, exclusively licensed to Novartis, Research Funding; Bristol-Myers Squibb: Other: PI or co-investigator on clinical trial(s) funded via contract with OHSU., Research Funding; Pfizer: Other: PI or co-investigator on clinical trial(s) funded via contract with OHSU., Research Funding; Merck & Co: Patents & Royalties: Dana-Farber Cancer Institute license #2063, Monoclonal antiphosphotyrosine antibody 4G10, exclusive commercial license to Merck & Co; Dana-Farber Cancer Institute (antibody royalty): Patents & Royalties: #2524, antibody royalty; Burroughs Wellcome Fund: Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Patents & Royalties, Research Funding. Byrd:Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau; Genentech: Research Funding; Acerta: Research Funding; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; Novartis: Other: Travel Expenses, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau; BeiGene: Research Funding; Ohio State University: Patents & Royalties: OSU-2S. Levine:C4 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Isoplexis: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Loxo: Membership on an entity's Board of Directors or advisory committees; Qiagen: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Prelude Therapeutics: Research Funding; Novartis: Consultancy; Gilead: Consultancy; Lilly: Honoraria; Imago Biosciences: Membership on an entity's Board of Directors or advisory committees. Mims:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Astellas Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; PTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal