Introduction: Isocitrate dehydrogenase 1 and 2 (IDH1/2) are metabolic enzymes in the citric acid cycle, producing alpha-ketoglutarate (αKG). Mutations in specific regions of these genes have been characterized in gliomas, AML and chronic myeloid neoplasms such as MDS and MPN. These mutations produce an oncometabolite 2-hydroxyglutarate (2HG) that disrupts the epigenetic phenotype of myeloid cells promoting oncogenesis. While this process has been characterized for arginine hot spot (R132/R140) mutations, there exist variants of unclear significance (IDHVUS), with potential pathogenic predictions using in silico assessment models. With the availability of mutant IDH inhibitors in practice, it has become important to characterize these VUS. We carried out this study to functionally interrogate potentially pathogenic IDHVUS in MDS and MPN.
Methods: After institutional approval, bone marrow (BM) DNA of patients with MDS and MPN, including overlap syndromes were subjected to next generation sequencing (29-gene, including the entire coding region of IDH1/2) by previously described methods (Patnaik et al., BCJ 2016). A sub-set of patients with available plasma [IDH wild type (IDHWT), bona fide IDH mutations (IDHMT) and IDHVUS with predicted pathogenicity (≤0.01% minor allele frequency, and predicted damaging in silico scores)] were submitted for mass spectrometry (LC-MS) based detection of IDH-related metabolites; D and L isomers of 2HG, αKG, and total 2HG. The impact of variations on protein secondary structure was assessed by the GorIV prediction algorithm. Potential alterations within the tertiary structure of the proteins was evaluated by in silico mutagenesis.
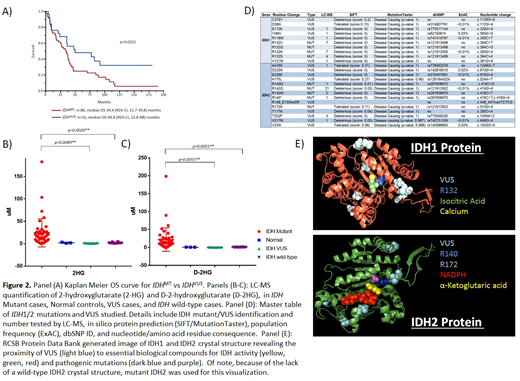
Results: One hundred and seventeen patients were included in the study; median age 68 years (range, 39-94), 68% male. Of these, 40 (34%) had MDS, 42 (36%) MPN, and 35 (30%) MDS/MPN overlap syndromes. Eighty six (74%) had IDHMT, including n=28 (24%) with IDH1MT (R132C/G/H/S) (11 MDS, 8 MPN, 9 MDS/MPN) and n=59 (50%) with IDH2MT (R140G/Q/W) (22 MDS, 20 MPN, 17 MDS/MPN). IDHVUS were detected in 32 (27%) patients, including 1 IDH1MT patient, and involved IDH1 in 14 (12%) cases and IDH2 in 18 (15%) cases. All IDHVUS were missense, except for a 2 amino acid insertion in IDH2, with the most frequent VUS being IDH1Y183C (7%) and IDH2T352P (7%).
While there were no phenotypic differences between IDH1/2MUT and IDH1/2VUS, in comparison to patients with IDH1/2MT, those with IDH1VUS were more likely to have higher WBC counts (median 8.1 vs 3.6 x109/L, p=0.0322), higher neutrophil counts (median 3.3 vs 1.3 x109/L, p=0.0203), and lower BM blast % (median 1 vs 3%, p=0.02061). Compared to IDH1/2MT patients, those with sole IDH1/2VUS more frequently had mutations in SF3B1 (p=0.0167), while they were associated with a lower frequency of SRSF2 mutations (p=0.0002). Interestingly, TET2 mutations were detected in only 5% of patients with IDHMT and 16% of IDHVUS patients, although without reaching statistical significance.
LC-MS analysis on 75 plasma samples (n=43 IDHMT, n=9 IDHVUS, n=20 IDHWT, 3 normal) revealed a significant increase in total 2HG levels in IDHMT vs IDHVUS (p=0.0089), and IDHWT (p=0.0020) cases (Figure 2A-D). While there were no significant differences in αKG levels between the groups, the D-2HG enantiomer levels were higher in IDHMT samples vs IDHVUS (p=0.0199) and IDHWT (p=0.0055). On examination of the crystal structures of the IDH genes using PDB, while we observed a direct interaction between R132/R140/R172 and the catalytic core, the IDHVUS were located away from this active center and were hence thought to be allosteric in nature (Figure 2F). These VUS however, did demonstrate predictive steric clashes in the tertiary structure context of IDH1 using the PyMol artificial mutagenesis tool. The absence of wild-type crystal structure for IDH2precluded a similar analysis for IDH2.
Median overall survival (OS) of the entire cohort (Figure 1) was 36 (range, 0-190) months, with OS being inferior in IDH1/2MT patients (34 months) vs those with IDH1/2VUS (50 months, p=0.0255).
Conclusion: Our study demonstrates that in spite of predictive pathogenicity of IDH1/2VUS using in silico predictive tools and crystal studies, the lack of involvement of the arginine hot spot precludes IDH neomorphic activity, as shown by normal 2HG levels. In addition, in comparison to IDHMT patients, those with IDHVUS had fewer BM blasts and a better survival.
Patnaik:Stem Line Pharmaceuticals.: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal